Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.3 Medellín Sept./Dec. 2011
PHARMACOLOGY AND TOXICOLOGY
INHIBITORY EFFECTS OF PLANT PHENOLIC COMPOUNDS ON ENZYMATIC AND CYTOTOXIC ACTIVITIES INDUCED BY A SNAKE VENOM PHOSPHOLIPASE A2
EFECTOS INHIBITORIOS DE COMPUESTOS FENÓLICOS DE PLANTAS SOBRE LA ACTIVIDAD ENZIMÁTICA Y CITOTOXICA INDUCIDA POR UNA FOSFOLIPASA A2 DE VENENO DE SERPIENTE
Jaime A. PEREAÑEZ1*; Vitelbina NÚÑEZ1,2; Arley C. PATIÑO1; Mónica LONDOÑO1; Juan C. QUINTANA1
1 Programa de Ofidismo/Escorpionismo. Universidad de Antioquia. A.A. 1226. Medellín, Colombia.
2 Escuela de Microbiología. Universidad de Antioquia. A.A. 1226. Medellín, Colombia.
* Corresponding autor: andres.pereanez@siu.udea.edu.co.
Received: 23 February 2010
Accepted: 25 April 2011
ABSTRACT
Polyphenolic compounds have shown to inhibit toxic effects induced by snake venom proteins. In this work, we demonstrate that gallic acid, ferulic acid, caffeic acid, propylgallate and epigallocatechingallate inhibit the enzymatic activity of a phospholipase A2 (PLA2), using egg yolk as substrate. The IC50 values are between 0.38 – 3.93 mM. These polyphenolic compounds also inhibit the PLA2 enzymatic activity when synthetic substrate is used. Furthermore, these compounds decreased the cyotoxic effect induced by a myotoxic PLA2; specifically, epigallocatechin gallate exhibited the best inhibitory capacity with 90.92 ± 0.82%, while ferulic acid showed the lowest inhibitory activity with 30.96 ± 1.42%. Molecular docking studies were performed in order to determine the possible modes of action of phenolic compounds. All polyphenols showed hydrogen bonds with an active site of enzyme; moreover, epigallocatechingallate presented the strongest binding compared with the other compounds. Additionally, a preliminary structure-activity relationship analysis showed a correlation between the IC50 and the topological polar surface area of each compound (p = 0.0491, r = -0.8079 (-0.9878 to -0.2593)), which indicates the surface area required for each molecule to bind with the majority of the enzyme. Furthermore, our results show that propylgallate and epigallocatechingallate are two novel natural products with anti-myotoxic potential. The topical application of these plant polyphenols at the bite site could lead to prevent myotoxicity; however, further in vivo studies would be necessary to confirm the in vitro results.
Key words: Snake bite, phenolic compounds, local tissue damage, phospholipase A2, molecular docking.
RESUMEN
Los compuestos fenólicos han mostrado inhibir los efectos tóxicos inducidos por proteínas de veneno de serpiente. En éste trabajo, nosotros demostramos que el ácido gálico, el ácido ferúlico, el ácido cafeico, el propilgalato y el epigalocatequingalato inhiben la actividad enzimática de una fosfolipasa A2 (PLA2) usando yema de huevo como sustrato. Los valores de IC50 están entre 0,38 – 3,93 mM. Los compuestos mencionados también inhiben la actividad enzimática cuando un sustrato sintético es usado. Además, estos compuestos polifenólicos disminuyen el efecto citotóxico inducido por la fosfolipasa A2 miotóxica, el epigalocatequingalato exhibe la mejor capacidad inhibitoria con 90,92 ± 0,82%, mientras que el ácido ferúlico muestra la menor actividad inhibitoria con 30,96 ± 1,42%. Con el fin de determinar los posibles mecanismos de acción de los compuestos fenólicos, realizamos estudios de modelamiento molecular. Todos los polifenoles muestran puentes de hidrogeno con el sitio activo de la enzima; además el epigalocatequingalato presenta una unión más fuerte con la PLA2 que los otros compuestos. Adicionalmente, un análisis preliminar de relación estructura actividad muestra una correlación entre los valores de IC50 y el área superficial polar topológica (p = 0,0491, r = -0,8079 (-0,9878 a -0,2593)), la cual indica el área superficial requerida por cada molécula para unirse a la enzima. Además, nuestros resultados muestran al propilgalato y el epigalocatequingalato como dos nuevos productos naturales con potencial antimiotóxico. La aplicación tópica de estos polifenoles en el sitio de mordedura podría llevar a la prevención de la miotoxicidad; sin embargo, posteriores investigaciones in vivo serían necesarias para confirmar los resultados in vitro.
Palabras clave: Accidente ofídico, compuestos fenólicos, daño tisular local, fosfolipasa A2, modelamiento molecular.
Key words: alpha.
INTRODUCTION
Snakebites represent a relevant public health issue in many regions of the world, particularly in tropical and subtropical countries of Africa, Asia, Latin America and Oceania (1). The pathophysiological effects observed in ophidian bites combine the action of several enzymes, proteins and peptides, which include phospholipases A2, hemorrhagic metalloproteases and other proteolytic enzymes, coagulant components, neurotoxins, cytotoxins and cardiotoxins, among others (2). Phospholipases A2 (PLA2; EC 3.1.1.4) are enzymes that abundantly occur in snake venoms with crucial action in the hydrolysis of phospholipids. PLA2 can also induce several pharmacological effects such as edema, modulation of platelet aggregation, as well as neurotoxic, anticoagulant and myotoxic effects (3, 4). To explain the susceptibility of a tissue to a particular PLA2 enzyme, the presence of ''target sites'' on the surface of target cells was proposed (3). These target sites are recognized by specific ''pharmacological sites'' on PLA2 molecules. These pharmacological sites are independent of, but sometimes overlapping with, the active site of the enzyme (3).
Myotoxic PLA2s bind to acceptors in the plasma membrane (target sites), which might be lipids or proteins, and which may differ in their affinity for the PLA2s. Upon binding, myotoxic PLA2s disrupt the integrity of the plasma membrane by catalytically dependent (phosphoipid hydrolysis) or independent mechanisms (interaction of pharmacological site with cell membrane). As a consequence, there is a loss in the control of permeability to ions and macromolecules. The most critical event is a prominent Ca2+ influx, which initiates a complex series of degenerative events associated with hypercontraction and mechanical damage of plasma membrane, activation of calpains and cytosolic Ca2+-dependent PLA2s, Z line loss, and mitochondrial Ca2+ overload (5). These events occur rapidly, provoking necrosis in muscle cells. The role of the catalytic activity in the induction of this effect depends of a particular enzyme. Therefore, alkylation of PLA2 by BPB, which is bound specifically in the His48 of the catalytic site, abolishes their enzymatic activity and reduces several pharmacological activities (anticoagulant, myotoxic, cytotoxic, edema-forming), suggesting their dependence on the integrity of this site. However, the effect of this modification on other pharmacological activities is less remarkable for some enzymes. These observations suggest that, despite the evidences of different sites, hydrolytic activity plays a considerable role in some biological effects (6).
The therapy for snakebites has been based on the intravenous administration of equine or ovine antivenoms (7). However, it has been demonstrated that this therapy generally has a limited efficacy against the local tissue damaging activities of venoms (8). Thus, there is a need to search for inhibitors and approaches that may be useful to complement conventional antivenom therapy.
Plant extracts constitute a rich source of pharmacologically active compounds, some of which have been reported to be an alternative to antagonizing the activity of various crude venoms and purified toxins (9-11). However, only a few of those chemical compounds have been isolated and identified as active components (12-14); from those compounds, a considerable number has been classified as polyphenols (15-17), which is a group of chemical substances found in plants and microorganisms, characterized by the presence of more than one phenol unit per molecule. Polyphenols are generally divided into hydrolyzable tannins (gallic acid esters of glucose and other sugars) and phenylpropanoids, such as lignins, flavonoids, and condensed tannins, among others. These compounds are one of the most versatile from the plant kingdom, they present effects such as the inhibition of HIV and the inhibition of human simplex virus (HSV), as well as antioxidant, bactericidal, antihelmintic, and antihepatoxic activities, among others (18).
Hence, the aim of this study was to demonstrate the inhibitory ability of the following phenolic compounds on the enzymatic and cytotoxic activities of snake venom PLA2: gallic acid, ferulic acid, caffeic acid, propylgallate, and epigallocatechingallate (shown in figure 1). For this purpose, we tested the inhibitory capacity of these compounds on PLA2 from the crotoxin complex (CB isolated from the Colombian Crotalus durissus cumanensis rattlesnake). This toxin is responsible for the neurotoxicity and local/systemic myotoxicity effects in the snakebite inflicted by this species. In order to determine the possible mode of action of these compounds; we have performed molecular docking studies and preliminary structure-activity relationship analysis.
MATERIALS AND METHODS
Chemicals and reagents
Caffeic acid, ferulic acid, propylgallate, gallic acid, tannic acid and epigallocatechingallate were purchased from Sigma and used without further purification. In all cases, compounds were diluted in 3% DMSO in PBS. The other reagents used in this work were purchased from Sigma and Merck, and their purity level was the highest available. Due to the capacity of tannic acid to precipitate proteins and its ability to inhibit snake venom proteins (19- 21), it was used as control for inhibition in all assays, and the other phenolic compounds were always compared with it.
Isolation of PLA2
Crotalus durissus cumanensis venom was obtained from four specimens maintained in captivity at the serpentarium of the Universidad de Antioquia (Medellin, Colombia). PLA2 was purified through molecular exclusion chromatography on Sephadex G-75 and reverse-phase HPLC on C-18 column eluted at 1.0 mL/min with a gradient from 0 to 100% of acetonitrile in 0.1% trifluoroacetic acid (v/v). The absorbance in the effluent solution was recorded at wavelength of 280 nm (21).
Inhibition of the phospholipase A2 activity using egg yolk as substrate
PLA2 activity was assayed according to the method established by Dole (22), with titration of free fatty acids released from egg yolk phospholipids, which were suspended in 1% Triton® X-100, 0.1 M Tris-HCl, 0.01 M CaCl2, pH 8.5 buffer, using 15 µg/10 µL of PLA2. The time of reaction was 15 min at 37°C. The protein sample was selected from the linear region of activity curves. For inhibition experiments, 0.5, 1, 2 and 4 mM of each compound were pre-incubated for 30 min at 37°C before the PLA2 activity determination. Results are indicated as inhibition percentage, where 100% is the activity induced by PLA2 alone. Tannic acid was taken as control for inhibition. The IC50 was determined from the linear portion of the response dose curves.
Inhibition of phospholipase A2 activity using 4-nitro-3-octanoyloxy-benzoic acid (4N3OBA) as substrate
The measurements of the enzymatic activity using the linear substrate 4N3OBA were performed according to the method described by Holzer and Mackessy (23), and adapted for a 96-well ELISA plate. The standard assay contained 200 µL of buffer (10 mM Tris-HCl, 10 mM CaCl2, 100 mM NaCl, pH 8.0), 20 µL of 10 mM of substrate (4NO3BA), 20 µL of the sample (20 µg PLA2 or 20 µg PLA2 + 2 mM of each compound), and 20 µL of water. The negative control consisted only of buffer. The inhibitory effect of the molecules on PLA2 activity was determined through the co-incubation of the enzyme with each compound for 30 min at 37°C. After the incubation period, the sample was added to the assay and the reaction was monitored at 425 nm for 40 min (at 10 min intervals) at 37°C. The quantity of chromophore released (4-nitro- 3-hydroxy benzoic acid) was proportional to the enzymatic activity, and the initial velocity (Vo) was calculated considering the absorbance measured right after 20 min. Tannic acid was used as control for inhibition.
Inhibition of cytotoxic activity
Cytotoxic activity of the purified PLA2 and its inhibition was assayed on murine myotubes obtained from C2C12 s keletal muscle myoblast (ATCC CRL-1772) grown in 96-well plates, as previously described (24). The toxin alone, or mixed with compounds at concentrations equi valent to IC50, obtained from PLA2 activity inhibition assays, was incubated for 30 min at 37°C. Then, aliquots of 150 µL (containing 20 µg of toxin + compounds diluted in Dubelcco's Modified Eagle's Medium) were applied to the cultures. After 3h at 37°C, a supernatant aliquot was collected for determination of lactic dehydrogenase activity (LDH; EC 1.1.1.27) released from damaged cells using a kinetic assay (Wiener LDH-P UV). Tannic acid was used as control for inhibition. Additional controls consisted of cells incubated with compounds in the absence of toxins. Results are shown as the percentage of inhibition, considering toxin and culture medium to be 100 and 0% of activity, respectively.
Molecular docking and physicochemical properties
Molecular docking was carried out using a Molegro Virtual Docker (MVD) (25). MVD is based on a differential evolution algorithm; docking scoring function, Escore; and the solution of the algorithm takes into account the sum of the intermolecular interaction energy between the ligand and the protein (Einter), and the intramolecular interaction energy of the ligand (Eintra). Compound structures were built and minimized by means of ChemSketch 12.0, a software from ACD/Labs and available at http://www.acdlabs.com/download/chemsketch/download.html. The structure of PLA2 (PDB code 2QOG) from Crotalus durissus terrificus that showed 57% of homology with the PLA2 from C. d. cumanensis (21), which was used in this study, was uploaded without water molecules. When necessary, bonds, bond orders, hybridizations, and hydrogen atoms were added, charges were assigned (a formal charge of +2 for Ca ion) and flexible torsions of ligands were detected. Then, an automatic procedure was used to detect possible binding cavities. During this process, the maximum number of cavities was fixed to 5, the grid resolution was 0.80 Å, and the probe size was 1.2 Å; while the other parameters were set to default. Two cavities were detected, and the cavity around the catalytic site (with a volume of approximately 80.38 Å3) was used for docking calculations using the MolDock- Optimizer as the search algorithm. During docking, the grid resolution was set to 0.3 Å, while the binding site radius was set to 14 Å. RMSD thresholds for multiple cluster poses was set at < 1.00 Å. The docking algorithm was set at a maximum of 1,500 iterations with a simplex evolution population size of 50 and a minimum of 10 runs. The ligand configurations with minor Escore were chosen, and a visual inspection of the interactions at the active site was performed and recorded. In order to perform a preliminary structure-activity relationship study, several physicochemical properties of each compound were obtained from Molinspiration by means of the ''Calculation of Molecular Properties and Drug-likeness'' tool, available at http://www.molinspiration.com/cgi-bin/properties.
Statistical analysis
In order to determine the IC50 of each compound in the inhibition of PLA2 activity assay, the lineal portion of dose-response curve was used, and a simple lineal regression analysis was performed. To determine the significant differences between compounds and tannic acid in the same assay, a two-way ANOVA was performed, followed by a Bonferron's test. To determine the significant differences between compounds and tannic acid in the cytotoxicity inhibition assay, an ANOVA was performed, followed by a Dunnett's test, and a difference was considered significant when p < 0.05. A non-parametric correlation was carried out using the Spearman method between the TPSA (Topological Polar Surface Area) and the PLA2 inhibition of each compound. In all cases, results are shown as the mean ± SEM of n indicated in each case.
RESULTS AND DISCUSSION
Myonecrosis is a commonly found in snakebites, and it is caused by PLA2, one of the most important and abundant muscle damaging components present in snake venoms. The action of these enzymes over membrane phospholipids includes the release of fatty acids such as the arachidonic acid, which is a precursor of pro-inflammatory eicosanoids (26); moreover, such degradation can lead to destabilization of the phospholipids bilayer (4).
Recently, we demonstrated that the PLA2 used in this study exhibited the above mentioned effects, among others (21). This enzyme is a component of the crotoxin complex of the Colombian Crotalus durissus cumanensis rattlesnake venom, which is a heterodimeric complex that is formed by a basic PLA2 known as CB, and an acidic nonenzymatic component known as crotapotin, which increases the pharmacological activity of PLA2 (acting as chaperone protein for the enzyme, preventing the binding of PLA2 to non-specific sites) (27, 28). Crotoxin is responsible for neurotoxicity, renal failure, edema, and local and systemic myotoxicity in cases of snakebites inflicted by the South American Crotalus durissus rattlesnake (29). However, CB alone also induces these effects, and it belongs to the group IIA PLA2s, which shares the general characteristics shown in figure 1F: the structure is formed by three long α helixes (two of which are antiparallel), two β wings and a calcium-binding loop (figure 1F). These proteins have a variable length ranging from 119 to 134 amino acids. Their antiparallel α helixes (residues 37-57 and 90-109, respectively) define the hydrophobic channel, with the assistance of the N-terminal helix (residues 1-12). This region leads the substrate to the active site, which is formed by four residues: His48, Asp49, Tyr52 and Asp99; from which, the combination of Asp49 with Tyr28, Gly30 and Gly32 forms the calcium-binding loop, which is responsible of coordinating the Ca2+ required during catalysis. In addition, there is an interfacial binding surface, which mediates the adsorption of the enzyme onto the lipid-water interface of the phospholipids membrane bilayer (3, 30).
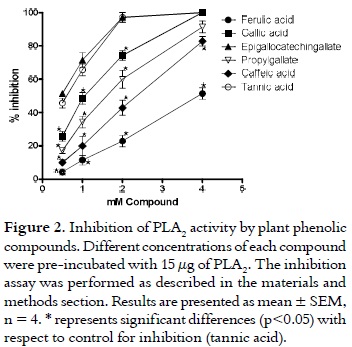
Tannins, the secondary metabolites of plants, are mostly water-soluble phenolic compounds that can produce the common phenolic reactions and can precipitate alkaloids, gelatin, and other proteins (18). According to their structures, tannins are categorized as hydrolysable tannins, condensed tannins, or complex tannins (18). Hydrolysable tannins are the esters of the 3, 4, 5-trihydroxyl benzoic acid (gallic acid), which are esterified to a core polyol, and the galloyl groups may be further esterified or oxidatively cross-linked to form more complex structures. The implication of the enzymatic activity is a key step in the induction of myonecrosis, inflammation and neurotoxicity induced by PLA2 (5, 31, 32). As it is shown in figure 2, tannic acid (control for inhibition) showed an excellent inhibitory capacity of PLA2 activity (IC50 = 0.59 mM). Likewise, epigallotechingallate exhibited similar inhibitory activity (IC50 = 0.38 mM). In addition, this compound did not reveal significant differences with respect to tannic acid (control for inhibition) at the concentrations used. At the highest concentration used, gallic acid and its derivative, propylgallate, did not exhibit significant differences with respect to control for inhibition. They presented the following IC50 values: 1.84 mM and 1.84 mM, respectively.
Contrastively, the cinnamic acid derivatives, ferulic acid and caffeic acid showed the lowest inhibitory ability. They exhibited significant differences with respect to tannic acid (control for inhibition) at all the concentrations used (p > 0.05). Additionally, these compounds presented the following IC50 values: 3.93 mM and 1.40 mM, respectively.
Similarly, all compounds inhibited PLA2 activity when the synthetic substrate (4N3OBA) was used. However, as it is shown in table 1, epigallocatechingallate presented the best inhibitory ability, while ferulic acid exhibited the lowest inhibitory capacity.
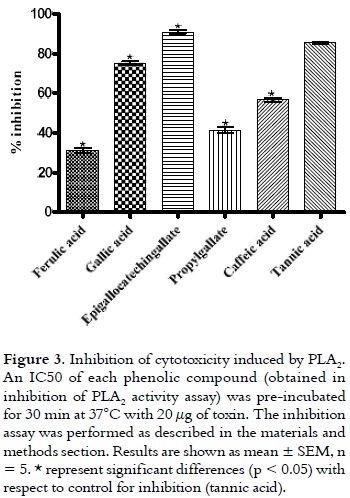
Due to the abundance of the PLA2 in the venom of viperids/crotalids, and to the large amount of venom injected during snakebite accidents, these myotoxins are undoubtedly central to the development of myotoxicity, which occurs in two clinical patterns: local and systemic myotoxicity (33). The action of PLA2 may result in irreversible lesions and even amputation of the affected limb (29). Additionally, it has been demonstrated that antivenoms generally have a limited efficacy against the local tissue damaging activities of venoms (7). Thus, there is a need to search for inhibitors and approaches that may be useful to complement conventional antivenom therapy. The use of cell cultures, such as rodent lines of skeletal muscle myoblasts/myotubes to evaluate miotoxicity of these PLA2 enzymes, appears to correlate well with their in vivo myotoxic activity (24). This correlation was used to demonstrate the inhibitory capacity of phenolic compounds on the cytotoxic activity induced by PLA2. As it is shown in figure 3, all compounds showed significant differences with respect to Tannic acid (control for inhibition).
However, the best inhibitory capacity was shown by epigallocatechingallate, with an inhibition of 90.92 ± 0.82 %. The lowest inhibitory activity was presented by ferulic acid with 30.96 ± 1.42 %; whereas, gallic acid, propylgallate and caffeic acid exhibited inhibition levels of 75.20 ± 0.75%, 41.34 ± 1.45%, and 56.50 ± 0.57%, respectively. At the concentration used, all compounds did not show cytotoxic effect on myotubes (data not show).
The non-selective precipitation on snake venom proteins and the chelating property of cofactors required by these enzymes are two possible modes of action attributed to polyphenols, especially those with complex structures (15, 16, 34, 35). However, by means of molecular docking studies it has been demonstrated that polyphenols (such as chlorogenic acid, curcumin, 1,3,5-trihydroxy benzene, 1,3-dihydroxy benzene and 2,4,6-trihydroxy acetophenone) inhibit PLA2 by interacting with the enzyme active site (36, 37). In order to explain the differences among the inhibitory effects induced by the polyphenols used in this study, a molecular docking analysis was performed. As it is shown in figure 4, all compounds could be perfectly adjusted in the active site of PLA2. In addition, all polyphenols showed a H-bonding interaction with Asp49. This bond could promote the destabilization of the calcium coordination, and it could cause a displacement of this cation from the calcium binding loop, which is essential for the enzymatic activity since it helps to polarize the sn2 ligation of the glycerophospholipids that will be hydrolyzed (27, 37). With the exception of propylgallate, all inhibitors presented a hydrogen bond with His48 that blocks water activation, which is important for a further basic general catalysis mechanism involved in hydrolysis of glicerophospholipids (30, 38). Additional H-bonding interactions were shown between caffeic acid and Cys29, and among epigallocatechingallate, Ala23 and Ala102. Moreover, the propylgallate carbonated side chain showed hydrophobic interactions with Leu6 and Phe5. Finally, as it is exposed in table 2, MolDock score values showed that epigallocatechingallate presented the strongest interaction energy, whereas gallic acid presented the least Escore (table 2). Nevertheless, this is a contradictory result, because gallic acid was the second more potent compound in the inhibition assays.
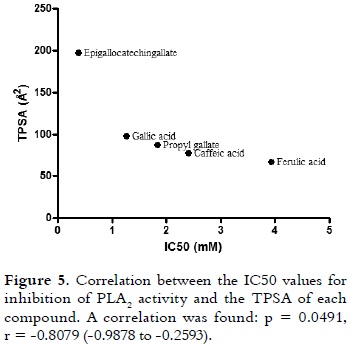
The best inhibitory ability of epigallocatechingallate on enzymatic and cytotoxic effects of PLA2 could be explained by its physicochemical properties, which are presented in table 1. TPSA is the sum of the contributions to the molecular (usually van der Waals) surface area of polar atoms, such as oxygen, nitrogen and their attached hydrogens (39). TPSA indicates the surface area required to bind with the majority of the target receptor (PLA2 in this case). As it is shown in figure 5, when a non-parametric correlation analysis between TPSA and IC50 values was performed, a significant correlation was observed (p = 0.0491, r = -0.8079 (-0.9878 to -0.2593)). Similar results were obtained with other polyphenolic compounds (flavonoids and isoflavones) for inhibiting telomerase and aromatase (40). The H bond donors and acceptors pattern of epigallocatechingallate is undoubtedly another property that should be considered. These donors and acceptors are known to play an important role in the contribution of water solubility by donning/accepting hydrogen bonds from water molecules and adding polarity to the structures. They also play an important role in drug-receptor interactions. Epigallocatechingallate has a great capacity of forming hydrogen bond interactions, providing a high affinity for PLA2 (as it is shown in table 2, Einter value). Therefore, this compound showed the lowest interaction energy (affinity) for the enzyme. And, this molecule also has four rotatable bonds, which may give more number of degrees of freedom for interacting with the PLA2, therefore, it has more possibilities to form a stable complex. In fact, this is supported by the highest value of internal energy shown by epigallocatechingallate (table 2).
Polyphenolic compounds, such as f lavonoids, have already been reported to be PLA2 inhibitors (41). Polyphenols (such as rosmarinic acid, aristolochic acid and α-tocoferol (vitamin E)) have also inhibited PLA2s from snake venoms (17, 42, 43). Moreover, ferulic acid, caffeic acid and gallic acid have shown an inhibitory ability against the activities induced by whole snake venoms (15, 44). However, these compounds had not been evaluated on purified PLA2s. Furthermore, from the results of this study, it can be concluded that propylgallate and epigallocatechingallate are two novel natural products with anti-myotoxic potential.
CONCLUSIONS
The use of plant extracts and other substances in different forms (poultices, steams, baths, among others) at the bite site is a common strategy used in the traditional medicine of several countries (10, 11). However, the efficacy of some of these practices have not been evaluated in controlled assays. In this study, some polyphenols that are present in different plants demonstrated to inhibit various activities induced by snake venom PLA2. The topical application of these plant polyphenols directly at the bite site should produce, to some extent, the desired anti-venom effects, particularly the prevention of myotoxicity, which generally cannot be cured through the administration of antivenom. However, further in vivo investigation is be necessary to confirm the in vitro results.
ACKNOWLEDGEMENTS
The authors would like to thank Paola Rey Suarez for her technical help in general. We are also very grateful to Dr. Rene Thomsen for granting us a trail license for the Molegro Virtual Docker, University of Aarhus, Denmark. This project was partly supported by Universidad de Antioquia and COLCIENCIAS (project 393-2006).
REFERENCES
1. Simpson ID, Norris RL. The global snakebite crisis-a public health issue misunderstood, not neglected. Wild Environ Med. 2009 Mar; 20 (1): 43-56. [ Links ]
2. Markland FS Jr. Snake Venoms. Drugs. 1997; 54 (Suppl 3): 1-10. [ Links ]
3. Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000 Oct 31; 1488 (1-2): 1-19. [ Links ]
4. Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003 Dec; 42 (8): 827-840. [ Links ]
5. Gutierrez JM, Ownby C. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003 Dec 15; 42 (8): 915-931. [ Links ]
6. Soares AM, Giglio JR. Chemical modifications of phospholipases A2 from snake venoms: effects on catalytic and pharmacological properties. Toxicon. 2003 Dec; 42 (8): 855-68. [ Links ]
7. Bon C. The serum - therapie was discovered 100 years ago. Toxicon. 1996 Feb 1; 34 (2): 142-143. [ Links ]
8. Gutierrez JM, Leon G, Rojas G, Lomonte B, Rucavado A, Chaves F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon. 1998 Nov; 36 (11): 1529-1538. [ Links ]
9. Martz W. Plants with a reputation against snake bite. Toxicon. 1992 Oct; 30 (10): 1131-1142. [ Links ]
10. Otero R, Nunez V, Jimenez S, Fonnegra R, Osorio R, Garcia ME, Diaz A. Snakebites and ethnobotany in the northwest region of Colombia. Part II: Neutralization of lethal and enzymatic effects of Bothrops atrox venom. J Ethnopharmacol. 2000 Aug; 71 (3): 505-511. [ Links ]
11. Coe FG, Anderson GJ. Snakebite ethnopharmacopoeia of eastern Nicaragua. J Ethnopharmacol. 2005 Jan 4; 96 (1-2): 303-323. [ Links ]
12. Nunez V, Castro V, Murillo R, Ponce-Soto LA, Merforf I. Inhibitory effects of Piper umbellatum and Piper peltatum extracts towards myotoxic phospholipases A2 from Bothrops snake venoms: isolation of 4-nerolidylcatechol as active principle. Phytochemistry. 2005 May; 66 (9): 1017-1025. [ Links ]
13. Soares AM, Ticli FK, Marcussi S, Lourenco MV, Januario AH, Sampaio SV, et al. Medicinal Plants with Inhibitory Properties Against Snake Venoms. Curr Med Chem. 2005; 12 (22): 2625-2641. [ Links ]
14. Marcussi S, Sant'Ana CD, Oliveira CZ, Rueda AQ, Menaldo DL, Beleboni RO, et al. Snake venom phospholipase A2 inhibitors: medicinal chemistry and therapeutic potential. Curr Top Med Chem. 2007; 7 (8): 743-756. [ Links ]
15. Pithayanukul P, Ruenraroengsak P, Bavovada R, Pakmanee N, Suttisri R, Saen-oon S. Inhibition of Naja kaouthia venom activities by plant polyphenols. J Ethnopharmacol. 2005 Mar 21; 97 (3): 527-533. [ Links ]
16. Leanpolchareanchai J, Pithayanukul P, Bavovada R, Saparpakorn P. Molecular docking studies and anti-enzymatic activities of Thai mango seed kernel extract against snake venoms. Molecules. 2009 Aug 27; 14 (4): 1404-1422. [ Links ]
17. Ticli FK, Hage LIS, Cambraia RS, Pereira PS, Magro AJ, Fontes MRM, et al. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): antiserum action potentiation and molecular interaction. Toxicon. 2005 Sep 1; 46 (3): 318-327. [ Links ]
18. Haslam E. Natural polyphenols (Vegetable tannins) as drugs: Possible modes of action. J Nat Prod. 1996 Feb; 59 (2): 205-215. [ Links ]
19. Pithayanukul P, Ruenraroengsak P, Bavovada, R, Pakmanee N , Suttisri, R. In Vitro. Investigation of the Protective Effects of Tannic Acid Against the Activities of Naja kaouthia. Venom. Pharm Biol. 2007 Feb 1; 45 (2): 94-97. [ Links ]
20. Kuppusamy UR, Das NP. Protective effects of tannic acid and related natural compounds on Crotalus adamenteus subcutaneous poisoning in mice. Pharmacol Toxicol. 1993 Apr-May; 72 (4-5): 290-295. [ Links ]
21. Pereanez JA, Nunez V, Huancahuire-Vega S, Marangoni S, Ponce-Soto LA. Biochemical and biological characterization of a PLA2 from crotoxin complex of Crotalus durissus cumanensis. Toxicon. 2009 Apr; 53 (5): 534-542. [ Links ]
22. Dole VP. A relation between non esterified-fatty acids in plasma and the metabolisms of glucose. J Clin Invest. 1956 Feb; 35 (2): 150-154. [ Links ]
23. Holzer, M, Mackessy, SP. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon. 1996 Oct; 34 (10): 1149-1155. [ Links ]
24. Lomonte B, Angulo Y, Rufini S, Cho W, Giglio JR, Ohno M, et al. Comparative study of the cytolytic activity of myotoxic phospholipases A2 on mouse endothelial (tEnd) and skeletal muscle (C2C12) cells in vitro. Toxicon. 1999 Jan; 37 (1): 145-158. [ Links ]
25. Thomsen R, Christensen MH. MolDock: a new technique for high accuracy molecular docking. J Med Chem. 2006 Apr 29; 49 (11): 3315-3321. [ Links ]
26. Murakami M, Kudo T. Secretory phospholipase A2. Biol Pharm Bull. 2004 Aug 10; 27 (8): 1158-1164. [ Links ]
27. Habermann E, Breithaupt H. The crotoxin complex: an example of biochemical and pharmacological protein complementation. Toxicon 1978; 16 (1): 19-30. [ Links ]
28. Hendon RA, Fraenkel-Conrat H. Biological roles of the two components of crotoxin. Proc Natl Acad Sci USA. 1971 Jul; 68 (7): 1560-1563. [ Links ]
29. Meier J, White J (Ed.). Handbook of Clinical Toxicology of Animal, Venoms and Poisons. Boca Raton, FL: CRC Press; 1995. Fan HW, Cardoso JLC. Clinical toxicology of snakebite in South America; p. 667-688. [ Links ]
30. Kini, R (Ed.). Venom phospholipase A2 enzymes: structure, function and mechanism. Chichester: John Wiley & Sons; 1997. Scott D. Phospholipase A2: structure and catalytic properties; p. 97-128. [ Links ]
31. Teixeira CF, Landucci EC, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003 Dec 15; 42 (8): 947-962. [ Links ]
32. Montecucco C, Gutierrez JM, Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell Mol Life Sci. 2008; 65 (18): 2897-2912. [ Links ]
33. Bon, C, Goyffon, M (Eds.). Envenomings and Their Treatments. Lyon: Fondation Marcel Merieux; 1996. Warrell, DA. Clinical features of envenoming by snake bites; p. 63-76. [ Links ]
34. Pithayanukul P, Leanpolchareanchai J, Bavovada R. Inhibitory effect of tea polyphenols on local tissue damage induced by snake venoms. Phytother Res. 2010 Jan; 24 (Suppl 1): S56-S2. [ Links ]
35. Pithayanukul P, Leanpolchareanchai J, Saparpakorn P. Molecular docking studies and anti-snake venom metalloproteinase activity of Thai mango seed kernel extract. Molecules. 2009 Aug 27; 14 (9): 3198-3213. [ Links ]
36. Nirmal N, Praba GO, Velmurugan D. Modeling studies on phospholipase A2-inhibitor complexes. Indian J Biochem Bio. 2008 Aug; 45 (4): 256-262. [ Links ]
37. Da Silva SL, Calgarotto AK, Maso V, Damico DCS, Baldasso P, Veber CL, et al. Molecular modeling and inhibition of phosp holipase A2 by polyhydro xy phenolic compounds. Eur J Med Chem. 2009 Jan; 44 (1): 312-321. [ Links ]
38. Berg OG, Gelb MH, Tsai MD, Jain MK. Interfacial enzymology: the secreted phospholipase A2-paradigm. Chem Rev. 2001 Sep; 101 (9): 2613-2654. [ Links ]
39. Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000 Oct 5; 43 (20): 3714-3717. [ Links ]
40. Doerksen RJ, Prasanna S. Topological Polar Surface Area: A Useful Descriptor in 2D-QSAR. Curr Med Chem . 2009; 16 (1): 21-41 [ Links ]
41. Lindahl M, Tagesson C. Flavonoids as phospholipase A2 inhibitors: importance of their structure for selective inhibition of group II phospholipase A2. Inflammation. 1997 Jun; 21 (3): 347-356. [ Links ]
42. Chandra V, Jasti J, Kaur P, Betzel Ch, Srinivasan A, Singh TP. First structural evidence of a specific inhibition of phospholipase A2 by alpha-tocopherol (vitamin E) and its implications in inf lammation: crystal structure of the complex formed between phospholipase A2 and alpha-tocopherol at 1.8 A resolution. J Mol Biol. 2002 Jul 5; 320 (2): 215-222. [ Links ]
43. Chandra V, Jasti J, Kaur P, Srinivasan A, Betzel Ch, Singh TP. Structural basis of phospholipase A2 inhibition for the synthesis of prostaglandins by the plant alkaloid aristolochic acid from a 1.7 A crystal structure. Biochemistry. 2002 Sep 10; 41 (36): 10914-10919. [ Links ]
44. Mors WB, Nascimento MC, Pereira BM, Pereira NA. Plant natural products active against snakebite-the molecular approach. Phytochemistry. 2000 Nov; 55 (6): 627-642. [ Links ]