Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.3 Medellín Sept./Dec. 2011
PHARMACEUTICAL INDUSTRY
PREPARATION AND PHYSICOCHEMICAL CHARACTERIZATION OF SOME POLYELECTROLYTE-DICLOFENAC COMPLEXES
PREPARACIÓN Y CARACTERIZACIÓN FISICOQUÍMICA DE COMPLEJOS POLIELECTROLITO-DICLOFENACO
Yolima BAENA A.1*; Rubén H. MANZO2; Luisa F. PONCE D'LEÓN Q.1
1 Grupo de Investigación en Sistemas de Liberación modificada de moléculas biológicamente activas (SILICOMOBA). Departamento de Farmacia, Universidad Nacional de Colombia. Carrera 30 No. 45-03 Bogotá- Colombia.
2 Departamento de Farmacia, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Edificio Ciencias 2, Ciudad Universitaria Córdoba-Argentina.
* Corresponding autor: ybaenaa@unal.edu.co.
Received: 20 October 2010
Accepted: 17 August 2011
ABSTRACT
The acid-base interaction between a polyelectrolyte and an ionisable drug could lead to the formation ionic complexes. The resulting physicochemical properties of such nanostructures could reflect changes in solubility, stability and drug-release pattern. This study was aimed to establish if diclofenac solubility could be modified by the formation of Eudragit® E and diclofenac polyelectrolyte drug complexes, which were prepared through the solvent evaporation method. The solid state was characterized by infrared spectroscopy and X-ray powder diffraction, and solubility was determined. The results suggested that complexes were formed in each case, they were called: EuD50, EuD50Cl10, EuD50Cl25, and EuD50Cl35. The drug apparent solubility presented values 431 and 1,498 times higher for EuD50Cl25 and EuD50Cl35 complexes respectively using water as solvent, and 3,674 and 10,412 times higher in physiological solution compared with free drug. It was concluded that the complexes have different solubility to the one of the parent drug and, therefore, they showed potential applications in the design of homogeneous liquid dosage forms.
Keywords: Complex, diclofenac, polymethylmethacrylate, solubility, ion pair.
RESUMEN
La interacción ácido-base entre un polielectrolito y un fármaco ionizable podría conllevar a la formación de complejos iónicos. Este tipo de nano-estructuras permitiría obtener propiedades fisicoquímicas diferentes a las del fármaco original, que se podría reflejar en variaciones de la solubilidad, de la estabilidad y del comportamiento de liberación del fármaco, entre otras. Este estudio se realizó para establecer si la solubilidad del diclofenaco podría verse modificada por la formación de complejos polielectrolito-fármaco con el Eudragit® E. Los complejos se elaboraron mediante el método de evaporación del solvente; se caracterizaron al estado sólido por espectroscopía infrarroja y difracción de rayos X y se les determinó la solubilidad aparente. Los resultados evidenciaron la formación del complejo en cada caso, denominados EuD50, EuD50Cl10, EuD50Cl25 y EuD50Cl35. Los valores de solubilidad aparente, fueron 431 y 1498 veces más altos para los complejos EuD50Cl25 y EuD50Cl35, respectivamente, cuando se empleó el agua como solvente; y 3674 y 10412 veces mayor en solución fisiológica, al compararlas con el fármaco sin complejar. Se concluyó que los complejos formados poseen solubilidades diferentes a la del fármaco original, con potencial aplicación en el diseño de formas farmacéuticas líquidas homogéneas.
Palabras Clave: Complejo, polimetilmetacrilato, diclofenac, solubilidad, par iónico.
INTRODUCTION
Complexes obtained from polymer associations have been of great interest in the pharmaceutical sciences. They are classified into different classes according to their type of association: metal ion complexes (i.e. inorganic, chelate, olefin and aromatic), organic molecular complexes (quinhydrone, picric acid, caffeine and other drug complexes and polymers), and inclusion compounds (e.g. cyclodextrines) (1). The polyelectrolyte-drug (PE-drug) complexes are thus classified to be from polymer type. Polyelectrolytes (PE) are chemical compounds that have been widely used in the drug formulation field, specifically for the acid-based ones (2). They are used and studied as viscosity-increasing agents in liquid and semisolid formulations, as well as components of modified conventional delivery systems. They are also used as basic drug carriers by the formation of polyelectrolyte-drug complexes, while basic PE are less used and have been slightly studied as acid drug carriers (1-5). Most of these studies have shown a PE influence on drug delivery properties (i.e. from the point of view of a particular system designed for such purpose); however, the drugs of interest in such studies have not been ionisable compounds having a chemical interaction with the PE in many cases, but they have been neutral molecules physically mixed with the polymer (2, 4). Hydrochloride diphenhydramine, hydrochloride verapamil, hydrochloride propanolol, and lydocaine have been some of the drugs used in these studies as models for complex formation. Fentanyl, estradiol and clonidine are other drugs that have been used in studies where their release was modified by the presence of a polymer (nonpolyelectrolyte) (1, 3).
A PE is a polymer that has acidic or basic groups in its structure, therefore, it has the ability to establish a chemical equilibrium between the dissociated and non-dissociated species in the solution (6). PE-drug complexes consist of a completely or partially neutralised PE containing an ionisable drug where chemical interactions predominate, thereby leading to the formation of ionic-pairs. Such complexes can be formed by different methods (7-10). Thus, some products having physicochemical properties significantly different to the ones of free drugs can be obtained regardless of the method used. Such variation in physicochemical properties can be reflected by changes in the solubility or improved drug compatibility (11), stability (12), and drug delivery behaviour (13). One of the most used methods has been the one known as solvent evaporation. It consists in dissolving the polyelectrolyte and the drug as neutral species to promote their acid-base interaction at a molecular level. Solvent evaporation is then facilitated by using a drying method (7).
This research work was focused on the PEdrug complex formation between a basic PE (Eudragit ® E) and an acid drug (diclofenac), and how this interaction can modify the solubility of the drug. Eudragit® E (Eu) is a cationic polymer based on dimethylaminoethyl methacrylate and other neutral methacrylic acid esters. The amino groups are protonated according to the pH, and they are positively charged below pH 5 (14). Diclofenac is a non-steroidal drug (NSAID) that has antiinflammatory, analgesic and antipyretic properties. In its acid form, it becomes different salts such as sodium, potassium and diethylamine. It is presented in several pharmaceutical dosage forms and it is administered by different routes (15). Several previous studies have described complex formation between cationic polymers and diclofenac, as well as several properties concerning the drug delivery from matrices (16, 17). One of the mentioned studies evaluated the ionized diclofenac's ability to interact with ammonium methacrylate copolymers (Eudragit® RL and RS) and its release pattern in different conditions (16). Another qualitative solubility study specifically dealt with Eu and anionic drugs by studying several complexes and evaluating the drug delivery properties of several dispersion complexes. The qualitative solubility of several acid molecules on forming complexes with Eu and 50% HCl, and the drug of interest were evaluated in the former study (16). An increase in the apparent solubility was found for each compound when HCl was present in most of them (8).
Therefore, this work was aimed to characterise some physicochemical properties of PE-drug complexes with diclofenac acid (DH), mainly its quantitative solubility. The objective was to suggest alternatives for improving the solubility of this drug (which was taken as a model due to its very low solubility in aqueous media), considering that the ionic-pair formation could improve the initial properties of the drug.
MATERIALS AND METHODS
Materials
The pharmaceutical grade sodium diclofenac (DNa) was a gift from Merck® Laboratories (Unique Chemicals. Batch: 327DFS17197). The diclofenac acid (DH) was then obtained from sodium diclofenac. Eudragit® E PO (Eu), pharmaceutical grade, poly (butyl methacrylate, (2-dimethyl aminoethyl) methacrylate, methyl methacrylate) 1:2:1, was a gift from Almapal®, Bogotá, Colombia (Röhn Pharma Polymers. Batch: G050731088; molecular weight: 150,000 daltons). Distilled water (< 2 μS cm-1 conductivity), hydrochloric acid (Mallinckrodt ® Chemicals), acetone (Merck® Chemicals), ethanol USP, 0.1N perchloric acid (Merck® Chemicals), glacial acetic acid (JT Baker® Chemical Co), sodium chloride (Carlo Erba® Reagents), and sodium hydroxide (Panreac® Química SA) were also used in this research.
Determining polymer amino group equivalents
Amino group equivalents per gram of Eu were assayed through acid-basic titration using 0.1 N perchloric acid, following the European Pharmacopoeia methodology (18).
Producing the free acid form of diclofenac
An aqueous solution of DNa was stoichiometrically acidified with 0.1 N hydrochloric acid and then crystallised in a water-ethanol solvent mixture (8). Then, the DH obtained was characterised through differential scanning calorimetry (Simultaneous Thermal Analyzer STA-Rheometric Scientific® system), at scan rate of 10°C/min, in a sealed crucible with a dynamic nitrogen flow.
Preparing solid complexes
Four complexes were prepared by means of the solvent evaporation method that was established in a previous study (7, 8). The equivalent of amino groups per gram of Eu was calculated for determining the amount of DH and 0.1 N HCl that should be added. The polymer (1g) and the appropriate amount of DH needed for neutralising 50% of the Eu amino groups were dispersed in 15 mL of acetone in the first complex. HCl was also added to the other three complexes (in different percentages) for neutralising the rest of the PE basic groups. After that, the solvent was evaporated in a vacuum oven at room temperature. The first complex was called EuD50 and the other PE-drug systems were classified as EuD50Cl10, EuD50Cl25 and EuD50Cl35.
Characterising complexes
EuDxClx complexes were characterised through infrared spectroscopy (IR) and X-ray powder diffraction to determine their chemical and crystallographic structure.
Infrared spectroscopy
An ATI Mattson Genesis Series spectrometer was used for the IR analysis on 1.5% PE-drug dispersed in KBr disks. The physical mixtures of Eu and DH, or just Eu or DH alone were also assayed.
X-ray powder diffraction
Solid products (EuDxClx), their respective physical mixture (Eu and DH), the polymer (Eu), and the drug (DH) were characterised in a 10°-70° 2θ/θ scan range with a scan speed of 0.066°2θ/s, using a Panalytical X'Pert PRO MPD system.
Quantitative evaluation of complex solubility
An excess of powder was introduced into flasks containing 10 mL of the solvent. The suspensions sealed in the flasks were first to be shaken in an ultrasonic bath and then transferred to another bath at 37°C ± 1°C. Manual agitation was required during the experiment. The excess solid was removed by means of filtration through a 0.45 μm pore size membrane after the phase equilibrium had been reached (72 hours). Liquid phase concentration was determined with an UV-Vis Biomate3 spectrophotometer (Thermo Electrocorporation, USA), at the maximum wavelength absorption previously selected for diclofenac (275 nm or 281 nm for DNa or DH, respectively).DH solubility was determined and compared to the apparent solubility (expressed as DH) of the four previously characterised complexes. The pH levels of the solutions were recorded.Water and 0.9% NaCl solution (physiological solution- PS) were the solvents used. All the results were the average of at least three independent measurements with their respective standard deviations. ANOVA was used for confirming differences or finding similarities in the results (α = 0.05).
RESULTS AND DISCUSSION
Determining polymer amino group equivalents
The amino group equivalent per gram of Eu, assayed through acid base titration, was 3.278 ± 0.035 meq/g. This value referred to the neutralisable basic groups of the polymer that are able to interact with the diclofenac carboxylic group. The amounts of DH and HCl required for preparing the complexes were calculated from this data.
Producing the free acid form of diclofenac
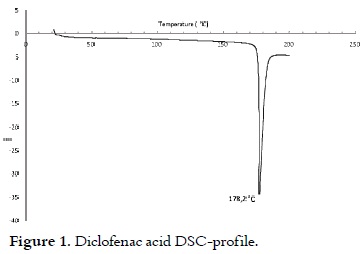
The obtained solid was characterised by DSC. Figure 1 shows the information provided by the DSC profile for DH. The endotherm of DH melting at 178.2°C can be observed in this profile, which is similar to the one reported by other authors in the same conditions (19).
Preparing solid complexes and characterising them
Four complexes were prepared in solid state (EuD50, EuD50Cl10, EuD50Cl25 and EuD50Cl35) and characterised as described below.
Infrared spectroscopy (IR)
IR has been used for studying chemical interactions at a molecular level in drug-polymer blends in the PE-drug complex field. This analysis was focused on the changes in bands associated with the diclofenac acid group and the polymer amino groups. If the drug and polymer ionically interacted in the complex, then the functional groups in the IR spectra would show the emergence of additional bands, alterations in the wave number position or broadening, compared with pure drug and polymer spectra (9).
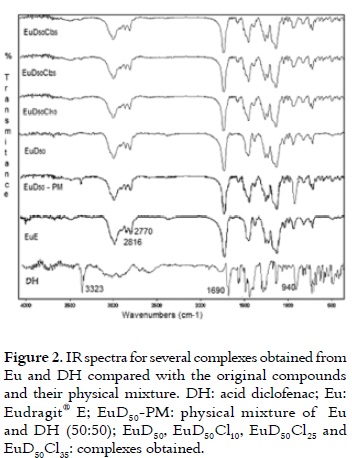
Figure 2 presents the IR spectra for DH, Eu, the physical mixture of DH and Eu (50:50), and the complexes. The diclofenac IR spectra showed characteristic bands at 3,300 cm-1, 940 cm-1, and 1,690 cm-1 that respectively represented the OH stretching associated with the carboxylic acid hydroxyl group, the OH vibrations out of plane from the same group, and the C-O stretching of carboxylic acid and carboxylate. The characteristic bands at 2,770 and 2,816 cm-1 for Eu IR spectra corresponded to dimethylamine groups. These bands were well-defined in the pure polymer and in the physical mixture. However, in the complexes, they decreased in intensity as the degree of polyelectrolyte neutralisation by DH was increased. The afore mentioned bands disappeared at 3,300 cm-1 and 940 cm-1, while the band at 1,690 cm-1 was displaced to the left.
These results provided a strong indication of the presence of ionic bonding between the Eu protonated dimethylamine groups and the DH carboxylate group in the different complexes.
X-ray powder diffraction
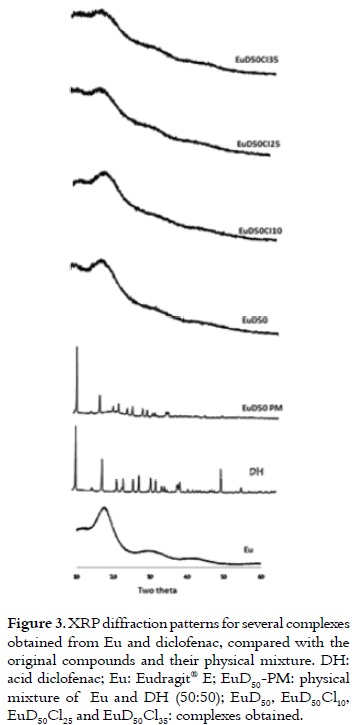
Figure 3 presents the XRPD profiles for the pure substances (DH and Eu), the physical mixture and the complexes that were obtained. The diclofenac XRPD profile showed many high intensity signals that are characteristic of a crystal structure, while Eu had a profile that is characteristic of an amorphous compound. The physical mixture presented a profile similar to the sum of the one for Eu and DH. The crystal signals for DH were not present when the complex was obtained and, therefore, it showed a profile that is characteristic of an amorphous compound. These results were in agreement with those observed by means of the IR.
Quantitative evaluation of complex solubility
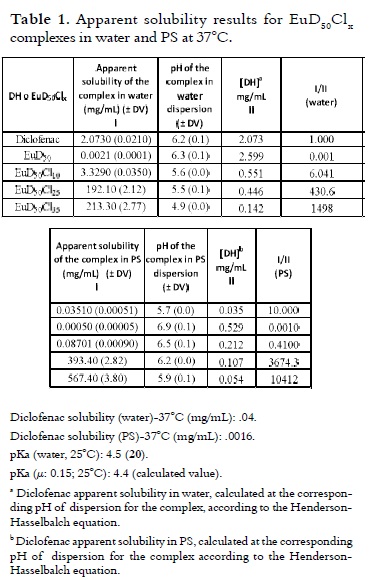
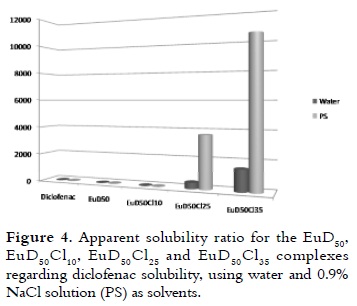
The results for the apparent solubility of the EuD50Clx complexes in water and PS at 37°C are illustrated in table 1. The intrinsic solubility of DH was also determined in the same conditions for comparison purposes. The solubility ratios (complex apparent solubility/diclofenac intrinsic solubility) are presented in figure 4.
Figure 4 shows the increased apparent solubility of the complexes compared with diclofenac intrinsic solubility. This pattern occurred because the drug was present as a free drug and also in ionic pairs with the PE (2, 5-6). This increase in apparent solubility was evident in the EuD50Cl25 and EuD- 50Cl35 complexes, but was not the same for EuD50 and EuD50Cl10, which apparent solubility was very low. As it has been described in other studies (5, 7), these types of PE-drug complexes were characterised in aqueous dispersions, revealing an important degree of counterion condensation between the macroion (PE), and the small counterions (D- and Cl-) due to the resulting electrostatic interaction.
The following chemical equilibriums were involved for the different species:
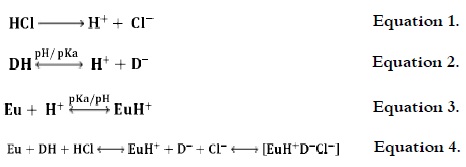
where Eu represents the neutral polymer; EuH+, the protonated polymer; DH, the diclofenac acid; D-, the dissociated diclofenac; and [EuH+ D-Cl-], the PE-drug complex.
According to the explanation provided by Quinteros et al., 2008 (7), the apparent solubility of a complex could be expressed in terms of different species in the solution, as follows:
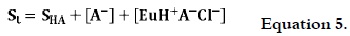
where SHA is diclofenac intrisic solubility, [A-] is the concentration of free dissociated diclofenac, and [EuH+A-Cl-] is the ion pair obtained between dissociated diclofenac, hydrochloric acid and Eu. It is known that at defined solvent (composition and pH) and temperature conditions, the drug concentration as non-dissociated and dissociated species is a constant value; therefore, the increase in apparent solubility will depend on the ionic-pair concentration in solution.
The results concerning water solubility, which are reported in table 1, showed different solubility values when the neutralisation degree was changed with HCl in the EuD50 complex series. Apparent solubility increased when the neutralisation percentage with HCl was higher in the complex. The opposite behaviour was occurred for the pH; when the degree of neutralisation was increased, the pH value tended to decrease. Thus, increased solubility in water could have been attributed to greater hydrophilicity in two complexes due to the higher polymer protonation. The EuD50Cl25 and EuD50Cl35 complexes presented this behaviour because the former reached levels 431 times greater than the intrinsic solubility of diclofenac at the same pH level, while the EuD50Cl35 complex was 1,498 times greater than the intrinsic solubility of diclofenac.
The apparent solubility of the complexes in PS was higher than their solubility in water, especially in the case of EuD50Cl25 and EuD50Cl35. This difference could have been due to Na+ and Cl- ions, which influence equilibrium, thereby promoting ion exchange:
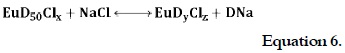
When equilibrium was displaced to the right (equation 6), a higher DNa concentration was obtained in the solution and, therefore, the pH value was increased (7) compared with the aqueous solutions of the complexes (table 1, pH changed as follows: 6.3 to 6.9; 5.6 to 6.5; 5.5 to 6.2 and 4.9 to 5.9). Table 1 shows the increased solubility for the EuD50Cl25 and EuD50Cl35 complexes compared with the solubility of diclofenac in PS. The solubility ratios obtained from these comparisons (presented in figure 4) showed that the apparent solubility of the complexes was 3,674 and 10,412 times greater than the solubility of diclofenac, being significantly more soluble than in water. Statistically significant differences were found by means of the ANOVA analysis (p < 0.05) between the results regarding complex and free drug solubility in the mediums used here (i.e. a drastic range of solubility in water and PS, and between the free drug and the complexes tested in this study).
CONCLUSIONS
IR and XRPD revealed that four complexes were actually formed. It was observed that when the degree of polymer neutralisation became increased with HCl in water and PS, the diclofenac solubility increased. EuD50Cl25 and EuD50Cl35 complexes had the highest solubility (431 and 1,498 times higher in water and 3,674 and 10,412 times higher in PS, compared with the solubility of diclofenac at the same pH) while EuD50 was the complex that has the lowest solubility in the solvents that were evaluated here. These results emphasised the change in apparent solubility when the complex became formed, and this interaction could have led to improving the compatibility of diclofenac with aqueous solvents when the solubility was increased. This is an important alternative to be considered when designing liquid dosage forms.
ACKNOWLEDGMENTS
The authors would like to thank the DIB (Universidad Nacional de Colombia - UNC) for providing financial support, and Universidad Nacional de Córdoba (UNALCO) (Argentina) for its academic support in this project. We would also like to thank the UNC's Pharmacy Department and the UNALCO's Pharmaceutical Technology Research Group (Argentina) for granting us access to the equipment and laboratories used in this research, as well as Mr. Jason Garry for painstakingly reviewing the manuscript.
REFERENCES
1. Sinko PJ. Martin's Physical Pharmacy and Pharmaceutical Sciences. 5th ed. Philadelphia, United States: Lippincott Williams & Wilkins; 2006. p. 615-618. [ Links ]
2. Florence AT, Attwood D. Physicochemical Principles of Pharmacy. 4th ed. London, United Kingdom: Pharmaceutical Press; 2006. p. 273-327. [ Links ]
3. Kim CJ. Controlled release dosage form design. Florida, United States: CRC Press; 2000. 194 p. [ Links ]
4. Aulton ME. Pharmaceutics. The Science of Dosage Form Design. 2nd ed. London, United Kingdom: Harcourt Publishers Limited; 2002. p. 295-302. [ Links ]
5. Jiménez-Kairuz A, Allemandi D, Manzo RH. Mechanism of lidocaine release from carbomer-lidocaine hydrogels. J Pharm Sci. 2002 Jan; 91 (1): 267-272. [ Links ]
6. Lankalapalli S, Kolapalli VRM. Polyelectrolyte complexes: A review of their applicability in drug delivery technology. Indian J Pharm Sci. 2009 Sep; 71 (5): 481-487. [ Links ]
7. Quinteros DA, Ramírez-Rigo V, Jiménez-Kairuz AF, Manzo RH, Allemandi DA. Interaction between a cationic polymethacrylate (Eudragit® E 100) and anionic drugs. Eur J Pharm Sci. 2008 Jan; 33 (1): 72-79. [ Links ]
8. Quinteros DA. Desarrollo de nuevas estrategias de formulación de fármacos mediante el acomplejamiento con polielectrolitos [dissertation]. [Córdoba]: Universidad Nacional de Córdoba; 2010. 184 p. [ Links ]
9. Takka S. Propranolol hydrochloride-anionic polymer binding interaction. IL Farmaco. 2003 May; 58 (10): 1051-1056. [ Links ]
10. Holgado MA, Fernandez-Arévalo M, Alvarez-Fuentes J, Caraballo I, Llera JM, Rabasco AM. Physical characterization of carteolol: Eudragit® L binding interaction. Int J Pharm. 1995 Jan 31; 114 (1): 13-21. [ Links ]
11. Vilches AP, Jimenez-Kairuz A, Alovero F, Olivera ME, Allemandi DA, Manzo RH. Release kinetics and up-take studies of model f luoroquinolones from carbomer hydrogels. Int J Pharm. 2002 Oct 10 (1-2); 246:17-24. [ Links ]
12. Jimenez-Kairuz AF, Allemandi DA, Manzo RH. The improvement of aqueous chemical stability of a model basic drug by ion pairing with acid groups of polyelectrolytes. Int J Pharm. 2004 Jan 9; 269 (1): 149-156. [ Links ]
13. Jimenez-Kairuz AF, Llabot JM, Allemandi DA, Manzo RH. Swellable drug-polyelectolyte matrices (SDPM). Characterization and delivery properties. Int J Pharm. 2005 Jan 6; 288 (1): 87-99. [ Links ]
14. Rowe RC, Sheskey PJ, Owen SC. Handbook of Pharmaceutical Excipients. London, United Kingdom: Pharmaceutical Press; 2006. p. 553-560. [ Links ]
15. Chuasuwan B, Binjesoh V, Polli JE, Zhang H, Amidon GL, Junginger HE, et al. Biowaiver monographs for immediate release solid oral dosage forms: Diclofenac sodium and diclofenac potassium. J Pharm Sci. 2009 Apr; 98 (4): 1206-1219. [ Links ]
16. Khalil E, Sallam A. Interaction of two diclofenac acid salts with copolymers of ammoniomethacrylate: effect of additives and release profiles. Drug Dev Ind Pharm. 1999 Apr 1; 25 (4): 419- 427. [ Links ]
17. Kim CJ. Advanced Pharmaceutics Physicochemical principles. Florida, United States: CRC Press; 2004. p. 453-455. [ Links ]
18. Directorate for the Quality of Medicines & Healthcare of the Council of Europe. European Pharmacopoeia. 4th ed. Strasbourg, France: Council of Europe; 2002. 1254 p. [ Links ]
19. Llinás A, Burley JC, Box KJ, Glen RC, Goodman JM. Diclofenac solubility: Independent determination of the intrinsic solubility of three crystal forms. J Med Chem. 2007 Mar 8; 50 (5): 979-983. [ Links ]
20. Martínez-Pla JJ, Escuder-Gilaber L, Sagrado S, Villanueva- Camañas RM, Medina-Hernández MJ. Chromatographic estimation of apparent acid dissociation constants (pKa) in physiological-resembling conditions. A case study: Ionisable non-steroidal anti-inf lammatory drugs. Internet Electron J Mol Des. 2005 Apr; 4 (4): 256-263. [ Links ]