Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.19 no.1 Medellín Jan./Apr. 2012
FOODS: SCIENCE, TECHNOLOGY AND ENGINEERING
Effect of pre- and postharvest application of 1-methylcyclopropene on the maturation of yellow pitahaya (Selenicerus megalanthus Haw)
EFECTO DE LA APLICACIÓN EN PRECOS ECHA Y EN POS COS ECHA DE 1-MCP EN LA MADURACIÓN DE PITAHAYA AMARILL A (Selenicerus megalanthus Haw)
Liliana SERNA C.1, Laura S. TORRES V.2, Alfredo A. AYALA A.2
1 Facultad de Ingeniería y Administración. Universidad Nacional de Colombia. Carrera 32 vía Candelaria, Palmira, Colombia. lserna@unal.edu.co.
2 Facultad de Ingeniería, Escuela de Ingeniería de Alimentos. Universidad del Valle. Calle 13 No. 100-00, Cali, Colombia.
Received: 03 June 2011
Accepted: 28 March 2012
ABSTRACT
The effect of pre- and postharvest applications of 200 and 400 μgL-1 aqueous solutions of 1-methylcyclopropene (1-MCP) on yellow pitahaya (maturity stage 3) was assessed. Changes in respiratory rate, color, firmness, soluble solids content, titratable acidity, maturity index, weight loss and commercial preservation were measured. Results showed that the preharvest application of 400 μgL-1 1-MCP enhanced fruit color, preserved firmness, and reduced weight loss and maturity index of fruits. It is suggested that these results were explained by the reduction of available membrane receptors able to bind ethylene, thus increasing ethylene sensitivity and response magnitude. The preharvest application of 1-MCP could potentially be used to accelerate pitahaya fruit ripening. The preharvest application of 200 μgL-1 1-MCP resulted in smaller changes in color and titratable acidity vs. control. Total soluble solids and pH were not significantly affected by the treatments. The postharvest application of 1-MCP maintained the texture of fruits by three additional days when compared to the untreated control.
Keywords: Tropical fruits, ethylene, respiratory rate, firmness, color.
RESUMEN
Se evaluó el efecto de la aplicación en precosecha y en poscosecha de soluciones acuosas de 200 y 400 μgL-1 de 1-MCP, con tiempo de exposición de 10 minutos, sobre la maduración de pitahaya amarilla entera en grado de madurez tres. Se midieron cambios en la respiración, pérdida de peso, color, firmeza, contenido de azúcares solubles, acidez, índice de madurez y tiempo de vida comercial. Los resultados demostraron que la aplicación de 400 μgL-1 de 1-MCP en precosecha acelera los cambios de color de la fruta, conserva su firmeza, disminuye la pérdida de peso y disminuye su índice de madurez. Lo anterior se explicó por la disminución en los receptores de membrana disponibles para interactuar con el etileno, lo que provoca incremento en la magnitud y sensibilidad de la respuesta del etileno. La aplicación de 1-MCP en precosecha podría tener aplicaciones en la aceleración de la maduración de frutas. La aplicación de 200 μgL-1 de 1-MCP en precosecha disminuyó los cambios de color y conservó la acidez. Los sólidos solubles, azúcares totales y pH no fueron influenciados significativamente por los tratamientos. La aplicación de 1- MCP en poscosecha mantiene la textura de la fruta durante 3 días más, comparada con el tratamiento control.
Palabras clave: frutas tropicales, etileno, tasa respiratoria, firmeza, color.
INTRODUCTION
Ethylene is a volatile hormone that affects the development of the entire plant (1), from seeds germination to leaves formation and growth, development and maturation of fruits, and plant senescence (2). The metabolic processes known as maturation are regulated by the binding of ethylene to a family of receptors with histidine kinase activity (3).
Ethylene analogs can compete for ethylene membrane receptors and modify the physiology of maturation and senescence, and also the preservation of fruits and vegetables (4). 1-methylcyclopropene (1-MCP) is a cyclopropene ethylene antagonist that exhibits excellent stability properties and effectiveness to control ethylene effects (5).
Additionally, 1-MCP inhibits the biosynthesis of ethylene by reducing the activity of the enzymes carboxylic acid synthetase (ACC synthase) and carboxylic acid oxidase (ACC oxidase) (6).
Previous studies reported the postharvest application of 1-MCP to control ripening and delay senescence of a large number of fruits, with results varying from delay to lack of fruit ripening. In most cases, the response to 1-MCP depended on concentration and moment of application, but varied for different species, in physiology and morphology of the fruit, variety, maturity stage, crop and storage conditions (7). The most common reported effects were the decay in softening and color development, reduction in respiratory activity and ethylene production, reduction in weight loss and fungi damage, and delay of processes related to ripening and senescence (7). A recent formulation (HarvistaTM, AgroFresh, Inc., Dow Chemical Co.) was developed for the preharvest application of 1-MCP in the field (8). This formulation improved fruit firmness and reduced the generation of ethylene, but did not have significant effect on fruit color, total soluble solids, and respiratory rate (8-10).
The use of pre- and postharvest applications of 1-MCP has not been studied in yellow pitahaya, an exotic cactus with worldwide flavor acceptance and good selling price in the international market (11). The pitahaya is a perennial plant with two harvest seasons (12) and only refrigeration has been studied to extend the commercial preservation of whole fruits.
Since enzymatic browning, skin necrosis, and skin softening are the main factors that negatively affect the commercial value of yellow pitahaya (13), it was hypothesized that the pre- and postharvest application of 1-MCP should favorably delay fruit softening, color development, weight loss, and other ripening related processes.
The objective of this study was to assess the effect of pre- and postharvest applications of 200 and 400 µgL-1 aqueous solutions of 1-MCP on the maturation of yellow pitahaya fruits. Fruit ripening was determined by the changes in respiratory rate, weight, color, firmness, soluble solids content, acidity, maturity index and commercial preservation.
MATERIALS AND METHODS
Plant material
Experiments were done on a 500 m2 parcel of yellow pitahaya (5000 plants/ha density) located in the municipality of Roldanillo, in the ''Valle del Cauca'' Department, Colombia (4° 24' 08¨ North latitude; 76° 09' 00¨ West longitude; 1450 m altitude). For the preharvest application, 15 days prior to collection, 80 fruits per treatment were randomly selected from the same row. For the postharvest application, fruits with a maturity stage 3 were selected. Maturity was determined using the NTC 3554 standard classification (14).
1-MCP preparation and application
A powder 1-MCP formulation (3.8%) obtained from Rohm and Haas (Philadelphia, Pennsylvania) was used. Solutions of 1-MCP at 200 and 400 µgL-1 in distilled water were prepared. For the preharvest experiments, the solutions were prepared in the field and applied by uniformly spraying the fruits on the trees, using a manual sprayer (2 bar operating pressure, 5 cm distance from the fruit). Fifteen days after application, the fruits were collected and rinsed in distilled water for one minute, dried outdoors, placed in plastic baskets (27 fruits per basket, arranged in a single layer), and stored in a controlled environment chamber (Model 1000L, Dies, Colombia) set at 25 ± 2°C and 75% relative humidity. For the postharvest application, the harvested fruit, with a maturity stage 3 (14), were first washed using distilled water and dried outdoors. Then, the fruits were submerged in aqueous solutions of 1-MCP with concentrations of 200 and 400 µgL-1 for 10 minutes, rinsed with distilled water, dried outdoors, placed in plastic baskets, and stored in the same conditions as described before. The same procedure was followed for the control treatment, using fruits with a maturity stage 3 but no application of 1-MCP. The following abbreviations will be used throughout the text: 200-PRE (200 µgL-1 preharvest application of 1-MCP), 400-PRE (400 µgL-1 preharvest application of 1-MCP), 200-POS (200 µgL-1 postharvest application of 1-MCP), 400- POS (400 µgL-1 postharvest application of 1MCP), and control (no application of 1-MCP).
Physiological, physical, and chemical analysis
Physiology of the fruits was evaluated by measuring respiratory rate. Physical properties were evaluated by measuring fruit weight loss, color, and firmness, and chemical properties by measuring soluble solids, and titratable acidity. All determinations were done in three replicates.
Respiratory rate measurement
The respiratory rate was measured by titration, using a modification to the method reported by Montes et al., 2001 (15), in figure 1. A refrigerated chamber externally equipped with a compressor and CO2 traps was used. Three hermetically sealed containers equipped with input and output hoses were placed inside the chamber. Each container held three previously weighed pitahayas. The compressor allowed external air to flow into a CO2 trap, containing 50 ml of 2N KOH to eliminate the CO2 present in the air by neutralization with a base. Then, the CO2-free air continuously flew to the hermetically sealed containers for 30 minutes. The air expelled from the desiccators, containing CO2 as a result of fruit respiration, was collected in secondary CO2 traps containing 50 ml of 0.1 N NaOH. The secondary CO2 traps were removed from the system and immediately sealed. Then, a 20 ml aliquot from each trap was mixed with 15 ml of 10% w/v BaCl2 (to form BaCO3), and 4 drops of phenolphtalein. The solution was immediately titrated with 0.1 N HCl using phenolphtalein as an indicator. Respiratory rate estimation was carried out daily during the first six days of storage, and then every three days. The respiratory rate (RR) expressed in mg of CO2 kg-1 h-1 was determined using equations 1 and 2.
Physical Properties
The wet weight of three pitahayas in each treatment was measured using a three-digit decimal precision scale (Mettler Toledo 1200, Columbus, Ohio). Then, the pitahaya fruits were placed in an environment controlled chamber (1000L Dies, Colombia) set at 25 ± 2°C and 85% relative humidity. Fruits were weighted daily during the first six days, and every three days subsequently. Weight loss was analyzed using the relative weight percent variation (ΔY) using equation 3.
Color was measured in the 400-700 nm reflectance spectrum using a Colorflex colorimeter (HunterLab, Reston, Virginia). Color coordinates L*a*b* for each fruit were obtained at five points in the equatorial zone, separated approximately by 72°, using the D65 standard illuminant and 10° observer. Three fruits per treatment were analyzed every three days for color determination. Color parameters were estimated by measuring total color change (ΔE) as shown in equation 4 (16).
Firmness was measured in three fruits per treatment every three days. Firmness was evaluated by measuring the maximum force required to penetrate 10.0 mm into the fruit, using a texture analyzer (model EZ-Test, Shimadzu, Somerset, New Jersey) equipped with a 2.0 mm diameter cylindrical penetrometer moving at 10 mm/min speed.
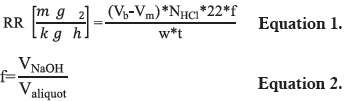
where: Vb = Volume (ml) of HCl used in blank titration; Vm = Volume (ml) of HCl used to titrate the sample; NHCl = Normality of HCl; w = simple weight (Kg); t = time (hours); 22 = milliequivalent weight of CO2 (g-meq); f = sample factor = (volume of NaOH / volume used for the sample).

where: Y0 = initial fruit weight; Yj = weight of fruit at time j.
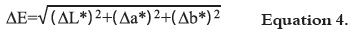
where: L* = Luminosity; a* = red to green color; b* = blue to yellow color.
Chemical Properties
The flesh from three pitahayas was mashed and used to determine total soluble solids (°Brix) and acidity. Total soluble solids were estimated using AOAC official method 932.12 by placing the liquid from the mashed fruit into a refractometer (Hand Held 500 HRS, Atago, Bellevue, Washington) (17).
Titratable acidity was measured using AOAC method 942.15A (18). NaOH 0.1 N was used to titrate to pH 8.1 and results were expressed as equivalent grams of citric acid.
The maturity index was determined as the ratio between total soluble solids and titratable acidity of fruit.
Evaluation of fruit preservation
Fruit preservation was determined by a daily evaluation of the physical appearance of the fruit and the quantification of the percent fruit that lost commercial value. The commercial preservation of individual fruit was defined as the number of days in storage before observing browning, necrosis of mammilla, or skin softening (firmness below 12 N). The percent loss was calculated as the ratio of damaged fruit over total fruit.
Experimental design
To determine the effect of the pre- and postharvest application of different 1-MCP aqueous solution on the ripening of fruit, a completely randomized factorial design with three factors and a control was used. Factors were moment of 1-MCP application at two levels (pre- and postharvest), 1-MCP concentration at two levels (200 and 400 µgL-1), and storage time at 10 levels (0, 1, 2, 3, 4, 5, 6, 9, 12, and 15 days). All determinations were done in triplicates.
Analysis of variance was done using the Minitab Statistical Program version 15.1 (Minitab, State College, Pennsylvania). When treatment effects were detected by ANOVA, multiple comparisons for the mean were done using the Tukey test (alpha=0.05).
RESULTS AND DISCUSSION
Respiratory rate
The respiratory rate of yellow pitahaya treated with 1-MCP aqueous solutions and the pre- and postharvest control treatments are shown in figure 2. All fruits exhibited a climacteric respiratory curve, in agreement with Rodriguez et al., 2005 (19), and Baquero et al., 2005 (20), and in contrast with reports by Nerd et al., 1997 (21, 22), who stored fruits at 20°C and found low CO2 generation and absence of a climacteric peak. The observed differences could be explained by differences in cultivar (23, 24), pre- and postharvest practices (24), and weather and agronomical conditions. Wills et al., 1998 (25) and Chitarra et al., 2005 (26), defined climacteric fruits as those exhibiting a sudden increase in the respiratory rate, which is typical of stored yellow pitahaya.
Preharvest application of 1-MCP enhanced fruit respiration. The maximum respiratory rate (38.3 mg CO2 kg-1h-1) was observed after four days of storage in fruit treated with 200 µgL-11-MCP, whereas the control treatment exhibited the lowest respiratory rate in figure 2a. The use of 1-MCP at 200 µgL-1 in postharvest displaced the climacteric peak to the sixth day of storage (26.9 mg CO2 kg-1h-1) and fruit receiving a postharvest application of 400 µgL-1 showed respiratory peaks during the second and sixth day of storage and lower respiratory rates that fluctuated between 5.9 and 14.60 mg CO2 kg-1h-1. Since the climacteric peak occurred during the initial days of storage, it was not related to the beginning of the senescence. However, it was considered to have an effect on the beginning or acceleration of the maturation metabolic processes (27).
The ANOVA showed a significant effect of storage time on the respiratory rate of yellow pitahaya fruit (p < 0.002). However, 1-MCP levels and time of application were not significant. These results were similar to reports by Dong et al., 2002 (28), where the application of 1000 nL L-1 of 1-MCP did not significant affect the respiratory rate of apricots. Dong et al., 2001 (29), also found similar results when 1 µgL-1 of 1-MCP was applied to nectarines. Defilippi et al., 2007 (8), found that the preharvest application of 1-MCP (HarvistaTM) did not affect the respiratory rate of apples. In this study, the observed respiratory rate values were lower than those reported by Rodriguez et al., 2005 (19), in yellow pitahaya.
Weight loss
All treatment factors had a significant effect on weight percent loss (p < 0.05; figure 3). The lowest weight percent loss was observed in fruit exposed to 400 µgL-11-MCP preharvest in figure 3a and the highest weight loss in fruit treated with postharvest 1-MCP applications. The postharvest use of 1-MCP resulted in similar weight percent losses of 15.6 and 16.3% for 200 and 400 µgL-1 1-MCP applications, respectively.
Nerd et al., 1997 (22), reported that yellow pitahaya exhibited a maximum 23% weight loss when stored at 20°C and 70% RH. These results were higher than those obtained in our study where control fruit showed a 15% maximum weight loss during storage. Weight percent loss in fruit after postharvest treatments in figure 3b was not in agreement with results by Massoloa et al., 2011 (30), where the postharvest application of 1-MCP in eggplant resulted in the lowest weight percent loss. Valero et al., 2007 (31), also observed the lowest weight percent loss in plums treated with postharvest 0.3 and 0.5 µgL-11-MCP and stored at 1 and 10°C. Other authors did not observed significant treatment effects for the case of apricots treated with 1-MCP (32).
The water balance in fruit is determined by the entrance of sap through the xylem and phloem and the loss of water by transpiration. Storage conditions play an important role in the fruit water balance since cuticular transpiration is determined by the difference between vapor pressure in the fruit surface vs. its surroundings (33). Transpiration by fruit during storage generates loss of water and therefore weight loss (34). Our results showed that the postharvest application of 1-MCP in yellow pitahaya caused a negative effect on weight, increasing postharvest economic losses.
Color
The major color changes in fruit were observed with the preharvest application of 1-MCP in figure 4, where the 200-PRE treatment resulted in the highest color percent change in figure 4a. Significant differences among control and treated samples (p < 0.05) were found after the sixth day of storage. At the end of the storage time, total changes in fruit color for the CONTROL, 200-PRE and 400-PRE treatments were 41.09, 52.68, and 42.31% respectively.
All treatments resulted in color changes after prolonged storage, but faster changes were observed in yellow pitahaya treated with preharvest 1-MCP. Gutierrez et al., 2008 (4), found similar behavior in pears, where fruit subjected to 1-MCP at concentrations of 0.4 and 0.8 µL L-1, showed the biggest change in luminosity and color. Their results were different from reports in the preharvest applications of 1-MCP (HarvistaTM) in apples (8, 9) and pears (10) where no significant differences in color were observed. Changes in fruit color are explained by the metabolism of green pigments and the exposure of typical previously masked colors of fruit in the early stage of maturity.
Fruit treated with postharvest applications of 1-MCP, showed minor changes in color (figure 4b). The average change in color (See equation 4) at the end of storage was 41.6, 19.22 and 28.69% for fruit treated with 0 (control), 200, and 400 µgL-1 postharvest 1-MCP, respectively. The postharvest application of 200 µgL-1 1-MCP yielded fruit with color attributes similar to freshly harvested fruit, but with longer storage preservation. Studies in broccoli (35), and grapefruit (36), reported that the postharvest application of 1-MCP delayed color changes in fruit, maintaining their characteristics during storage. It was also reported that postharvest applications of 1-MCP in watermelon retarded adverse changes in color, pH, and soluble solids content caused by ethylene (37). Although ANOVA showed that concentration of 1-MCP, storage time, and moment of application had all significant effect on total color change (p < 0.05), the last two factors were the most important in determining total color change.
Firmness
All yellow pitahaya fruits (treated and controls) exhibited firmness losses during storage. However, faster loss of firmness was observed in control vs. treated fruit in figure 5. In fruit treated with preharvest 1-MCP applications, firmness remained similar to that of freshly harvested fruit for up to 12 days of storage in figure 5a. The preharvest application of 1-MCP (HarvistaTM) retained firmness in apples (8, 9) and pears (10). The decline in firmness occurred earlier in fruit receiving postharvest 1-MCP applications, with significant differences between treated and control samples observed by the 15th day of storage in figure 5b.
Fruit treated with 400-PRE showed an 18.27% relative firmness loss during storage time vs. 19.26% in fruit treated with 200-PRE. Significant differences in firmness were observed due to moment of 1-MCP application (p < 0.019), and storage time (p < 0.001). Similar results were observed in tomatoes by Mostofi et al., 2003 (38), where the application of 250 nL L-1 of 1-MCP reduced firmness loss. Similarly, 1-MCP at 20-30 nL L-1 concentration levels, reduced the softening of plums harvested at two maturity stages (31).
The firmness of fruit is related to cell properties including adhesion between neighboring cells, cell brittleness, and cell turgor pressure (39). The cell wall is mainly formed by rigid cellulose microfibers held together by a network of glucans (hemicelluloses) and pectin arrays, and small quantities of structural proteins and aromatic compounds (40). During ripening, various enzymes degrade pectin and glucan arrays, affecting the structural integrity of the cell wall (41).
The hydrolysis and degradation of structural polymers, accumulation of osmotic solutes in the cell wall (41, 42), environmental conditions affecting the turgidity (42), and loss of water during ripening (41, 43) are all factors potentially affecting the texture of fruit by promoting changes in cell dimensions, intercellular adhesion, and cell wall composition (41). The use of 1-MCP reduces the activity of enzymes promoting pectin and hemicelluloses degradation, resulting in less significant changes in fruit firmness during storage (23).
Soluble solids
The soluble solids content in control samples varied from 17.1 oBrix at day one after harvest to 16.4 °Brix after 15 days of storage, and exhibited a peak of 18.4 °Brix on the fourth day of storage. These results differ from experiments by Nerd et al., 1997 (22), who found that yellow pitahaya fruits harvested green and mature exhibited constant soluble solids contents that ranged from 19 to 21 °Brix when stored at 10 and 20°C.
Fruit treated with preharvest applications of 1-MCP showed overall reductions on soluble solids during storage in figure 6a. The control and 400- PRE treatments exhibited an increase in soluble solids up to the second day, but subsequent reductions during storage. The reduction in soluble solids was coincident with the beginning of the respiratory climacteric peak. In contrast, a constant reduction in soluble solids during storage was observed in fruit treated with 200-PRE. Similar behavior was evident when fruit was treated with postharvest applications of 1-MCP in figure 6b.
The observed reductions in soluble solids are mainly explained by the use of simple sugars trapped inside vacuoles as substrates for respiration, via the glycolytic, pentose phosphate, or tricarboxylic acid pathways (44). The oscillating variation in soluble solids may be due to the existence of a different sugar source available for respiration as reported by Nerd et al., 1997 (21). Similar behavior was reported in onions by Chope et al., 2007 (45). Rodriguez et al., 2005 (19), measured the °Brix in yellow pitahaya fruits (maturity stages 3 and 5) and reported soluble solids between 14 and 16 °Brix for fruit stored at 19 °C and fluctuating values from 12 and 18 °Brix when fruit was stored at 8 °C. These values differed from our results and those reported by Nerd et al., 1997 (22).
The postharvest applications of 1-MCP generated a strong variability in soluble solids in figure 6b. Although significant differences (p < 0.001) were found due to moment of application, concentration and storage time. A similar behavior was observed in onions treated with 0.1 µL L-11-MCP vs. untreated controls, where soluble solids yielded significant treatment effects (45). Previous experiments also showed significant effects due to the application of 1-MCP in pineapple (35).
Acidity
All studied factors had a significant effect on the total titratable acidity (TTA) of yellow pitahaya fruits. The postharvest application of 1-MCP resulted in the least reduction in TTA in fruit up to the 12th day of storage. However, at the end of the storage time, lower TTA was observed in treated vs. control samples in figure 7a. Although control and 1-MCP treated fruit exhibited the same trend towards the reduction in total titratable acidity, sharp peak variations were observed during storage. These peaks were mostly evident in fruit receiving postharvest applications of 1-MCP and during the 2nd and 10th days of storage in figure 7b.
Titratable acidity of pitahaya decreases because organic acids are metabolized during the storage of fruit. Citric acid is metabolized to sugars, aminoacids, and non volatile organic acids that are used in oxidative reactions during maturation (46). The sudden increases in TTA could be explained by citric acid synthesis from glucose during the Krebs cycle (47). Rodriguez et al., 2005 (19), harvested yellow pitahaya fruits with maturity stage 3 and observed that TTA ranged from 1.5 to 2.54% after 15 days of storage at 19°C and from 1.3 to 3% after 23 days of storage at 8°C. According to these authors, fruit harvested with maturity stage 5 and stored at 8 and 19 °C showed negligible changes in TTA during storage.
Our results agreed with data reported by Servarajah et al., 2001 (35), who found that the application of 1-MCP delayed the reduction of ascorbic acid content in stored pineapples. Dong et al., 2002 (28), reported similar behavior in plums and Bassetto et al., 2005 (48), found high total acidity levels in 1-MCP treated guava fruits stored at 25°C, due to the delay in the maturation process. It must be noted that the effect of the 1-MCP is limited in advanced stages of ripening (32) and studies in apricots showed no effect of 1-MCP treatments on the total acidity and soluble solids in mature fruit (28).
Maturity index
All studied factors promoted increments in the maturity index of yellow pitahaya fruits. Control fruit exhibited 115 and 200 maturity index at the beginning and end of the storage period, respectively. Fruit treated with preharvest application of 1-MCP showed initial and final maturity index of 123 and 174 for 200-PRE and 116 and 153 for 400-PRE treatments. Postharvest applications of 200 µgL-1 and 400 µgL-1 1-MCP resulted in final maximum maturity index of 181 and 162, respectively. The maturity index was significantly affected by the moment of application and the concentration of applied 1-MCP (p < 0.05). It also showed significant changes due to the maturation process during storage. The maturity index followed the pattern control-fruit > fruit with postharvest applications of 1-MCP > fruit treated with preharvest applications of 1-MCP. Valero et al., 2007 (31), found that the maturation of plums stored at 20°C was delayed by increasing concentrations of 1-MCP, which in turn improved the fruit organoleptic properties.
Fruit quality preservation
Treated samples were evaluated for their overall appearance to verify that the whole fruit was suitable for consumption. The control fruit was acceptable for consumption for up to 12 days, after which basal decay, browning, and peduncle detachment were observed. Rodriguez et al., 2005 (19), reported that yellow pitahaya fruit harvested at maturity stage 3 and stored at 19 °C retained their quality for 15 days. Likewise, Janick et al., 2008 (49), reported high quality preservation of yellow pitahaya fruits stored for 14 days at 10°C or 17 days at 5°C. The postharvest application of 200 µgL-1 1-MCP delayed in three days the signs of aging in fruit. After 15 days of storage, 47.3% of the treated fruit lost their commercial quality vs. 84.2% for control fruit. Fruit receiving the postharvest application of 400 µgL-1 1-MCP showed signs of aging after 12 days of storage and 50% of the fruit lost their commercial value after 15 days of storage. Although control fruit and fruit receiving the preharvest application of 1-MCP (200 and 400 µgL-1) simultaneously began their commercial decay at day 12 of storage, 68% of fruit treated with 400 µgL-1 lost their commercial value by day 15.
A series of physical, chemical, and physiological changes occur in the fruit after harvesting due to the sudden end of water and solids circulating from the plant into the fruit (33) and the increase in activity of hydrolytic enzymes generating plant metabolites. The application of 1-MCP inhibits the production of ethylene (6) and the interaction between ethylene and its receptors (5). However, in preharvest applications of 1-MCP, metabolic processes responsible for shorten the yellow pitahaya preservation are triggered. This is because the magnitude and sensitivity to ethylene rises as a lower number of receptors are present (2, 3). The increase in the ethylene sensitivity together with the production of ethylene by untreated fruit and plant organs increase the response of stored fruit to the hormone.
CONCLUSIONS
The pre- and postharvest applications of 200 and 400 µgL-1 1-MCP aqueous solutions affected the preservation of the yellow pitahaya fruits during storage. The physical, physiological, and chemical effects depended on the concentration of the compound, the moment of application, and the storage time. Preharvest applications of 200 and 400 µgL-1 1-MCP could be used to accelerate external changes in yellow pitahaya fruits by promoting epicarp coloration, maintaining firmness, and delaying weight loss and maturity index. However, the maximum concentration of 1- MCP used in preharvest (400 µgL-1), yielded a high percent of pitahaya fruits showing undesirable signs of senescence.
The postharvest application of 200 µgL-11-MCP extended the commercial preservation of pitahaya fruits in three days by reducing maturity index, color changes, and fluctuations in titratable acidity. In contrast with similar reports in tropical fruits, our experiments indicated that postharvest applications of 1-MCP promoted greater weight losses in fruit during storage. Total soluble solids were not significantly affected by the use of 1-MCP.
ACKNOWLEDGMENTS
The authors acknowledge the support received from the Ministry of Agriculture and Rural Development, and the Association of Pitahaya Growers (Asoppitahaya).
REFERENCES
1. Guo H, Ecker JR. The ethylene signaling pathway: new insights. Curr Opin Plant Biol. 2004 Feb; 7 (1): 40-49. [ Links ]
2. Binder BM. The ethylene receptors: Complex perception for a simple gas. Plant Sci. 2008 Jul-Aug; 175 (1-2): 8-17. [ Links ]
3. Adams-Phillips L, Barry-Giovannoni J. Signal transduction systems regulating fruit ripening. Trends Plant Sci. 2004 Jul; 9 (7): 331-338. [ Links ]
4. Gutierrez MS, Trinchero GD, Cerri AM, Vilella F, Sozzi GO. Different responses of goldenberry fruit treated at four maturity stages with the ethylene antagonist 1-methylcyclopropene. Postharvest Biol Tec. 2008 May; 48 (2): 199-205. [ Links ]
5. Sisler EC. The discovery and development of compounds counteracting ethylene at the receptor level. Biotechnol Adv. 2009 Jul-Aug; 24 (4): 357-367. [ Links ]
6. Khan AS, Singh Z. 1-MCP application suppresses ethylene biosynthesis and retards fruit softening during cold storage of 'Tegan Blue' Japanese plum. Plant Sci. 2009 Apr; 176 (4): 539-544. [ Links ]
7. Watkins CB. The use of 1-methylcyclopropene (1-MCP) on fruit and vegetables. Biotechnol Adv. 2006 Jul-Aug; 24 (4): 389-409. [ Links ]
8. Defilippi BB, Campos R, Manríquez D. Uso de 1-Metilciclopropeno (1-MCP) en Precosecha retarda avance de madurez en manzana. Aconex. 2007 Jul-Dic; 96: 5-8. [ Links ]
9. Elfving DC, Drake SR, Reed AN, Visser DB. Preharvest applications of sprayable 1-methylcyclopropene in the orchard for management of apple harvest and postharvest condition. Hort Science. 2007 Aug; 42 (5): 1192-1199. [ Links ]
10. Moggia YJ. Preharvest use of 1-MCP (HarvistaTM Technology) in orchards: Effect on storage quality of Packham's Triumph Pears. Hort Science. 2008; 43 (4): 1088-1088. [ Links ]
11. Nerd A, Sitrit Y, Kaushik RA, Mizrahi Y. High summer temperatures inhibit flowering in vine pitahaya crops (Hylocereusspp.). Sci Hortic. 2002 Dec; 96 (1-4): 343-350. [ Links ]
12. Boletín CCI. Perfil de producto: pitahaya [Internet]. Bogotá. (Col): Corporación Colombia Internacional, Sistema de Inteligencia de Mercados; 1999. No. 5. [Revisión: 2010 Deciembre 3; Citacion 2011 Febrero 16]. Disponible en: http://www.cci.org.co/cci/cci_x/Sim/Perfil%20de%20Productos/perfilpitahaya5.htm. [ Links ]
13. Castro JA, Baquero LE, Narvaez CE. Catalase, peroxidase and polyphenoloxidase from pitahaya amarilla fruits (Acanthocereus pitajaya). Rev Colomb Quim. 2006 Jun; 5 (1): 91-100. [ Links ]
14. Instituto Colombiano de Normas Técnicas y Certificación. Norma Técnica Colombiana. NTC 3554. Frutas frescas. Pitahaya Amarilla. Bogotá: ICONTEC 1996. p. 1-14. [ Links ]
15. Montes J, Arévalo S. Determinacion del calor de respiración de frutas por el método de titulación. RAIA. 2001; 2 (1): 27-37. [ Links ]
16. Manresa A. El color en la industria de alimentos. 2da ed. Vicente I. La Habana, Cuba: Universitaria; 2007. 69 p. [ Links ]
17. Official methods of analysis of the Association of Official Analytical Chemists. AOAC 932.12 Fruits and fruit products. Solids (Soluble) in Fruits and Fruit Product: Refractometer Method. Arlington: 2000. p. 7. [ Links ]
18. Official methods of analysis of the Association of Official Analytical Chemists. AOAC 942.15A Fruits and fruit products - Acidity (Titratable) of Fruit Products. Association of Official Analytical Chemists, Arlington: 2000. p. 11. [ Links ]
19. Rodríguez DA, Patiño MP, Miranda D, Fischer G, Galvis JA. Efecto de dos índices de madurez y dos temperaturas de almacenamiento sobre el comportamiento en poscosecha de la pitahaya amarilla. Rev Fac Nal Agr Med. 2005 Jul-Dic; 58 (2): 2837-2857. [ Links ]
20. Baquero LE, Castro JA, Narváez CE. Catalasa, Peroxidasa y Polifenoloxidasa en pitahaya amarilla (Acanthocereus Pitajaya): maduración y senescencia. Acta Biol Colomb. 2005; 10 (2): 49-59. [ Links ]
21. Nerd A, Mizrahi Y. Reproductive biology of cactus fruit crops. Hort Rev. 1997; 18: 321-346. [ Links ]
22. Nerd A, Mizrahi Y. The effect of ripening stage on fruit quality after storage of yellow pitahaya. Postharvest Biol Tec. 1997 Feb; 15 (2): 99-105. [ Links ]
23. Liou S, Miller W. Factors affecting ethylene sensitivity and 1-MCP response in tulip bulbs. Postharvest Biol Tec. 2011 Mar; 59 (3): 238-244. [ Links ]
24. Watkins CB. Overview of 1-Methylcyclopropene trials and uses for edible horticultural crops. Hort Science. 2008 Feb; 43 (1): 86-94. [ Links ]
25. Wills RBH, LeeTH, Graham D, McGlasson WB, Hall EG. Postharvest: an introduction to the physiology and handling of fruits and vegetablesand ornamentals. 4th ed. New York, EEUU: CAB International, New York; 1998. [ Links ]
26. Chitarra MIF. Pós-colheita de frutas e hortaliças: fisiologia e manuseio. 2da ed. Chitarra AB. Sao Paulo, Brasil: UFLA; 2005. 785 p. [ Links ]
27. Bower J, Holford P, Latche A, Pech JC. Culture conditions and detachment of the fruit influence the effect of ethylene on the climacteric respiration of melon. Postharvest Biol Tec. 2002 Sep 1; 2 (2): 135-146. [ Links ]
28. Dong L, Lurie S, Zhou HW. Effect of 1-methylcyclopropene on ripening of 'Canino' apricots and 'Royal Zee' plums. Postharvest Biol Tec. 2002 March; 24 (2): 135-145. [ Links ]
29. Dong L, Zhou HW, Sonego L, Lers A, Lurie S. Ethylene involvement in the cold storage disorder of 'Flavortop' nectarine. Postharvest Biol Tec. 2001 Nov; 23 (1): 105-115. [ Links ]
30. Massoloa J, Concellóna A, Chavesa A, Ariel R, Vicente, A.1- Methylcyclopropene (1-MCP) delays senescence, maintains quality andreduces browning of non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol Tec. 2011 Jan, 59 (1): 10-15. [ Links ]
31. Valero D, Martinez-Romero D, Valverde JM, Guillén F, Serrano M. Quality improvement and extension of shelf life by 1-methylcyclopropene in plum as affected by ripening stage at harvest. IFSET. 2007 Sep; 4 (3): 339-348. [ Links ]
32. Fan X, Argenta L, Mattheis JP. Inhibition of ethylene action by 1-methylcyclopropene prolongs storage life of apricots. Postharvest Biol Tec. 2000 Sep; 20 (2): 135-142. [ Links ]
33. Leonardi C, Baille A, Guichard S. Predicting transpiration of shaded and non-shaded tomato fruits under greenhouse environments. Sci Hortic. 2000 Jun; 84 (3-4): 297-307. [ Links ]
34. Rizzini FM, Bonghi C, Tonutti P. Postharvest water loss induces marked changes in transcript profiling in skins of wine grape berries. Postharvest Biol Tec. 2009 Jun; 52 (3): 247-253. [ Links ]
35. Selvarajah S, Bauchot AD, John P. Internal browning in coldstored pineapples is suppressed by a postharvest application of 1-methylcyclopropene. Postharvest Biol Tec. 2001 Nov 23; (2): 167-170. [ Links ]
36. Mullins ED, McCollum TG, McDonald RE. Consequences on ethylene metabolism of inactivating the ethylene receptor sites in diseased non-climacteric fruit. Postharvest Biol Tec. 2000 Jun; 19 (2): 155-164. [ Links ]
37. Saftner R, Luo Y, McEvoy J, Abbott JA, Vinyard B. Quality characteristics of fresh-cut watermelon slices from non-treated and 1-methylcyclopropene- and/or ethylene-treated whole fruit. Postharvest Biol Tec. 2007 Apr; 44 (1): 71-79. [ Links ]
38. Mostofi Y, Toivonen PMA, Lessani H, Babalar M, Lu CW. Effects of 1-methylcyclopropene on ripening of greenhouse tomatoes at three storage temperatures. Postharvest Biol Tec. 2003 Mar; 27 (3): 285-292. [ Links ]
39. Harker FR, Elgar HJ, Watkins CB, Jackson PJ. Hallett IC. Physical and mechanical changes in strawberry fruit after high carbon dioxide treatments. Postharvest Biol Tec. 2000 Jun; 19 (2): 139-146. [ Links ]
40. McCann MC, Carpita NC. Designing the deconstruction of plant cell walls. Curr Opi Plant Biol. 2008 Jun; 11 (3): 314-320. [ Links ]
41. Brummell DA. Cell wall disassembly in ripening fruit. Funct Plant Biol. 2006 Feb 3; 33 (2): 103-119. [ Links ]
42. Almeida DPF, Huber DJ. Apoplastic pH and inorganic ion levels in tomato fruit: A potential means for regulation of cell wall metabolism during ripening. Plant Physiol. 1999 Mar; 105 (3): 506-512. [ Links ]
43. Saladie M, Matas AJ, Isaacson T, Jenks MA, Goodwin SM et al. A Reevaluation of the Key Factors That Influence Tomato Fruit Softening and Integrity. Plant Physiol. 2007 Jun; 144 (2): 1012-1028. [ Links ]
44. Paliyath G, Murr DP, Handa AK, Lurie S. Postharvest biology and technology of fruits, vegetables, and flowers. New York, EEUU: Wiley-Blackwell, New York. 2008. [ Links ]
45. Chope GA, Terry LA, White PJ. The effect of 1-methylcyclopropene (1-MCP) on the physical and biochemical characteristics of onion cv. SS1 bulbs during storage. Postharvest Biol Tec. 2007 May; 44 (2): 131-140. [ Links ]
46. Davies JN, Maw PJ. Metabolism of citric and malic acids during ripening of tomate fruit. IFSET. 1972 Aug; 23 (8): 969-976. [ Links ]
47. Sweetman C, Deluc LG, Cramer GR, Ford CM, Soole KL. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry. 2009 Jul-Aug; 70 (11-12): 1329-1344. [ Links ]
48. Bassetto E, Jacomino AP, Pinheiro AL, Kluge RA. Delay of ripening of 'Pedro Sato' guava with 1-methylcyclopropene. Postharvest Biol Tec. 2005 Mar; 35 (3): 303-308. [ Links ]
49. Janick J. The encyclopedia of fruit & nuts. Paull RE. London, Inglaterra: CAB International; 2008. 915 p. [ Links ]














