Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.19 no.1 Medellín Jan./Apr. 2012
BIOTECHNOLOGY
BIOTECHNOLOGICAL APPLICATIONS AND POTENTIAL USES OF THE MUSHROOM TRAMESTES VERSICOLOR
APLICACIONES BIOTECNOLÓGICAS Y USOS POTENCIALES DEL HONGO TRAMESTES VERSICOLOR
Ketty A. CÓRDOBA M.1; Alicia RÍOS H.1*
1 Grupo de Investigación en Valoración y Aprovechamiento de la Biodiversidad. Universidad Tecnológica del Chocó ''Diego Luís Córdoba''. Ciudadela Universitaria, Barrio Nicolás Medrando. Cra.22 Nº 18b - 10, Edificio 11 oficina 301, Quibdó, Chocó.
* Corresponding author: aliciar@utch.edu.co.
Received: 21 June 2010
Accepted: 19 April 2012
ABSTRACT
The use of products obtained from fungi (particularly mushrooms) has increased lately due to their broad applicability in different scientific and industrial fields. The genus Trametes comprises a group of white rot producing ligninolytic fungi, with medicinal properties, biotechnological importance and environmental applications. One of the most potentially useful species is T. versicolor, formerly known as Coriolus versicolor or Polyporus versicolor. Also known as Yun Zhi in China, is a fungus species belonging to the class Basidiomycetes, which has a widespread application as medicinal mushroom, and is also consumed as food and tea infusion. It also produces extracellular enzymes such as laccases, manganese peroxidases, lignin peroxidases and H2O2 producing oxidases. These enzymes are capable of degrading such complex compounds as lignin, as well as certain industrial contaminants. T. versicolor is one of the first higher fungi to be used in the production of an approved medicine. In effect, polysaccharides like Polysaccharide Krestin and Polysaccharide Peptide obtained from the liquid fermentation of its mycelium, exhibit anticarcinogenic properties, and are therefore used for preparing several pharmaceutical products. Both polysaccharides are highly effective in treatments against cancer. The present review addresses certain properties of T. versicolor, as well as its potential applications in different biotechnological fields.
Keywords: Biotechnology, enzymes, fungi, polysaccharides, immunological tests..
RESUMEN
El uso de productos obtenidos a partir de hongos (especialmente setas) se ha incrementado últimamente debido a su amplia aplicación en diferentes campos científicos e industriales. El género Trametes comprende un grupo de hongos ligninolíticos productores de pudrición blanca, con propiedades medicinales, importancia en biotecnología y aplicaciones ambientales. Una de las especies potencialmente más útil es T. versicolor, antes conocido como Coriolus versicolor o Polyporus versicolor. También conocido como Yun Zhi en China, es un hongo perteneciente a las especies de la clase Basidiomicetes, presenta una generalizada aplicación como hongo medicinal, y también es consumido como alimento y como infusión de té. Esta especie además produce enzimas extracelulares como lacasas, manganeso peroxidasas, lignina peroxidasas y oxidasas productoras de H2O2. Estas enzimas son capaces de degradar compuestos tan complejos como la lignina, así como ciertos contaminantes industriales. T. versicolor es uno de los primeros hongos superiores utilizado en la producción de un medicamento aprobado. En efecto, los polisacáridos como polisacárido Krestin y polisacáridos péptidos obtenidos de la fermentación líquida de su micelio, presentan propiedades anticancerígenas, por lo que son utilizados para la preparación de varios productos farmacéuticos. Ambos polysacchapolisacáridos son muy eficaces en los tratamientos contra el cáncer. La presente revisión aborda ciertas propiedades de T. versicolor, así como sus posibles aplicaciones en diferentes campos de la biotecnología.
Palabras clave: Biotecnología, enzimas, hongos, polisacáridos, pruebas inmunológicas.
INTRODUCTION
The use of products obtained from fungi (particularly mushrooms) has been progressively increasing due to their medicinal, biotechnological and environmental applications. Among these species, a promissory one is T. versicolor (L.:Fr.) Pilát, formerly C. versicolor or P. versicolor (family Polyporaceae). Commonly known as ''Turkey Tail'', it is broadly distributed around the world, from temperate zones to subtropical forests. It holds an outstanding place among the Basidiomycetes that provoke white rot (lignin degraders), and is considered an important link in the ecological functioning of the forest as a hard wood primary decomposer (a recycler of dead trees) (1). T. versicolor (L.:Fr.) Pilát, also known as Yun Zhi in China has received increasing attention from the researchers in food and pharmaceuticals, and is one of the few medicinal mushrooms to have had its clinical effects extensively validated (2). Among all the fungi that are currently used for their medicinal properties, T. versicolor (L.: Fr.) Pilát is one of the best studied species when compared to other common ones, including shiitake (Lentinus edodes) or reishi (Ganoderma lucidum). Polysaccharides obtained from its mycelium, especially PSK (Polysaccharide Krestin) and PSP (Polysaccharide Peptide), have been studied in clinical tests with humans, exhibiting a wide variety of immunological effects both in vivo and in vitro. These extracts are commercially produced and frequently prescribed together with chemotherapy in Japan (3-8).
Diverse species of Basidiomycetes have been studied in recent years, due to their ability to degrade lignin and phenolic compounds. As one of the most studied ligninolytic fungi, T. versicolor is also used for delignification (9, 10) due to a non-specific oxidative process that includes three different ligninolytic enzymes from its catalytic system (laccase, lignin peroxidase and manganese peroxidase). The non-specificity of these enzymes is of great importance for degrading products that are structurally similar to lignin, such as Polycyclic aromatic hydrocarbons (PAH's), polychlorobiphenyls (PCB's) or polychlorinated biphenyls, Dichloro Diphenyl Trichloroethane (DDT) and azoic colorants, among others (11-13). This has determined the importance of these enzymes for biotechnological applications like bioremediation; and their potential of use in industry for treating sewage effluents, specifically for discoloring those coming from textile and paper plants (14-16).
Fungi generally are a key element in biodiversity, such as lignin-degrading organisms that play an important ecological role as they recycle nutrients within the ecosystem. But also play an important role in the life of man as protein source, production of secondary metabolites and enzymes obtained from other applications. In the Department of Chocó, since 2000 there have been some important reports on the study of fungi; from Mycological explorations carried out in areas near the city of Pretoria and other towns, among which may be mentioned: Tutunendo, Pacurita, Yuto (Atrato) and Cabo corrientes (Chocó), specific work such as (17- 19) have contributed to the knowledge of this tropical mycobiota unknown to the world. T. versicolor (L.: Fr.) Pilát is a fungal species of basidiomycetes that is emerging as promising resource of the mycobiota for economic development in our region for its wide distribution and abundance. Considering the studies by various authors (2, 3, 5, 7, 9, 11, 13, 15, 20-26), it is shown that this fungus has a wide variety of applications and potential uses in the field of medicine and environmental and industrial biotechnology. With the aim of highlight the different properties of T. versicolor (L.: Fr) Pilat, with respect to their biotechnological applications and potential use, was held this review for a period of six months between september 2007 and february 2008, which was updated during march and april, 2012.
Medicinal properties
T. versicolor (L.:Fr.) Pilát contains a mix of complex polysaccharides and polypeptides of high molecular weight. In Japan it is known as a biological response modifier. Indeed, numerous research studies have demonstrated an elevated production of gamma-interferon and interleukin-2, as well as the proliferation of T-cells, in the presence of purified fractions of the fungus. T. versicolor has shown antimicrobial, antiviral and antitumor properties, as well as hepatoprotective effects. These features have been attributed to the protein-bound polysaccharide - PSK aforementioned, which is currently in use in Japan as a cancer treatment, in conjunction with surgery, chemotherapy and/or radiation (5-7).
Diverse fungi are available for medical use. More than 50 species have shown anticarcinogenic activity both in vitro and in animal models; as well as in human cancer. The two proteoglycans obtained from T. versicolor - PSK and PSP - are the most promising substances. Both products have been object of phases II and III studies in China, where significant survival effects have been observed in cases of esophageal cancer (10).
Made up of 90% polysaccharides and 10% peptides, and obtained from the cultured mycelium of T. versicolor, PSP is an ample spectrum biological response modifier. This substance exhibits immunomodulating and antitumor activities with low cytotoxicity. It has been used in Asia, especially in China, as a co-adjuvant in the clinical treatment of cancer, specifically for stimulating the immunological condition of patients under chemo and/or radio therapy. Besides, PSP shows analgesic, antiviral and antibacterial effects (6, 7).
According to research carried out by different authors (5-8), T. versicolor immunonutrition or supplementation (fungi nutrition) has a significant effect on cancer cells, as far as it reduces telomerase activity and enhances the immune response of the organism, which shifts to the TH1 cell mediated mode, the most efficient antitumor response (5, 6). Likewise, T. versicolor supplementation can have a beneficial impact on patients with Leaky Gut Syndrome (LGS) (10) or CFS (Chronic Fatigue Syndrome), determining improvements with regards to both immune response and viral levels. This treatment has the potential to play a significant role in dealing with CFS, as well as other chronic viral diseases (10, 27).
Preliminary research indicates that T. versicolor can also determine significant benefits in the veterinarian care of cats and dogs. Good results have been reached in treating domestic animals affected by fibrosarcoma (dogs with prostate tumors), demonstrating that this is an important nutrition method for supporting the immune response of small domestic animals. Preliminary reports by veterinarians of the United Kingdom on the use of T. versicolor in tests with cats and dogs affected by different tumors (including stomach, colorectal, esophageal and nasopharyngeal cancer) reveal that these animals experienced remarkable improvements in energy, wellbeing, appetite and life quality (10, 27).
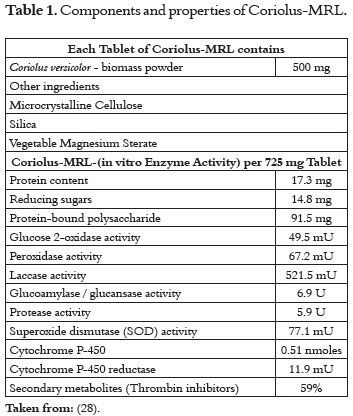
There is a commercial product in the market known as ''Coriolus-MRL®'', which is promoted as an immune system supporter. MRL is a T. versicolor powder containing both mycelium and primordia (parts of the fruiting body). It is processed under the same rigorous control conditions of conventional pharmaceutical products, which guarantee that the powder has not been contaminated by other fungi, heavy metals or pesticides. Thus, each tablet contains 500 mg of the standardized T. versicolor product, which is currently being largely commercialized at the international level. Its beneficial effects have been demonstrated in medical treatments with patients suffering different chronic diseases, who were supplied with the product (28). Table 1 describes the properties of the product.
A human dose of three grams of this product per day has been standardized, for periods ranging from 28 days to three years. T. versicolor extracts can be taken in infusion, capsules or pills. To some animals they have been injected. Although secondary effects are rare, some patients have reported stomach or intestine problems. Ames' test has not reported any genetic damage (27). According to studies carried out by Chinese researchers, PSP and PSK are relatively non-toxic products, both in the short and long term (6-8).
Enzyme production
One of the most important industrial uses for T. versicolor (L.:Fr.) Pilát is the production of enzymes such as Lignin peroxidase (LiP), Manganese peroxidase (MnP) and Laccase (LAC) (all of them ligninolytic agents from the complex enzymatic system of the fungus), mainly intended for the degradation of lignocellulosic material (11, 13, 29). The chemical unspecificity and intense oxidative activity of these enzymes confers them a remarkable capability of degrading different organic compounds with a similar structure to that of the monomeric units of lignin. Among the different products that can be transformed by the enzymatic activity of T. versicolor, we can mainly count xenobiotic compounds, pesticides, aromatic hydrocarbons (benzo[a]pyrene, pyrene, fluorene, phenanthrene, anthracene, catechol, dibenzothiophene), chlorinated organic compounds (pentachlorophenols, chloroanilines, polychlorinated biphenyls) atrazine, etc (11-19, 30, 31).
Laccase (benzenediol: oxygen oxidoreductase, EC 1.10.3.2): T. versicolor has been reported to be an excellent producer of this enzyme (32), which reduces molecular oxygen to water, and through the use of certain redox compounds can enhance its substrate spectrum, thus attaining the oxidation of the non-phenolic portions of lignin (33). The global reaction catalyzed by this enzyme is: 4 AH + O2 = 4A + 2H2O. The oxidized enzyme attacks mainly phenolic substrates, but in the presence of 1-hidroxibenzotriazole (1-HBT), 2,2'-azino-bis [3-ethylbenzthiazoline-6-sulfonic acid] (ABTS) or violúric acid , it is cooxidized and reaches the ability to degrade harder-to-oxidize non-phenolic substrates such as those found in paper pulp, chlorophenols, aromatic polyciclic hydrocarbons, kraft lignin, lignocellulosic substrates, etc (34-36).
Some of the laccase treatments have been potentiated by applying culture mediators or inductors such as 1Hid ro xibenzotriazole, 2,5-xylidine, veratryl alcohol (3,4- dimetoxibencil alcohol) and ethanol. This demonstrates that the production of laccase can be considerably increased due to the fact that some of these compounds are capable of stabilizing proteins, thus prolonging their activity (37-39).
The introduction of the concept of laccase mediators (based on the use of low molecular weight redox mediators), which produce fixed radicals, has broadened the potential application of laccases, which has therefore attained the pulp and paper industry (delignification and bleaching). These mediators increase the oxidative potential of the enzyme, thus conferring it the capability of oxidizing non-phenolic lignin. In these processes, immobilization methods are more effective than free cell culture medium methods, due to the biomass increase that results from the intense activity of laccase (40-44). The successful use of laccase in these processes requires large volumes of the enzyme, which, up to date can only be obtained through culturing (41, 42).
Manganese peroxidase (MnP, E.C. 1.11.1.13): Is an enzyme secreted to aid lignin degradation, catalyzing the chemical reaction that oxidizes numerous phenolic compounds, especially syringyl (3, 5-dimethoxy-4-hydroxyphenyl) and vinyl side-chain substituted substrates in the presence of Mn2+. (45) MnP oxidizes Mn2+ to Mn3+. The latter is chelated by organic acids that are synthesized by the fungus. That is, MnP can get to oxidize phenolic substrates through Mn2+, which is H2O2 dependent; but it can also attack non-phenolic substrates through products of peroxidation of unsaturated fatty acids (46).
Lignin peroxidase (LiP, E.C.1.11.1.14): Is a hemeprotein, can directly oxidize non-phenolic aromatic substrates through endogenous synthesis of H2 O2, being veratryl alcohol the most studied one. The reaction produces aryl and alkyl radicals, which are anabolized intracellularly. LiP is involving in the oxidative cleavage of non-phenolic aromatic lignin moieties and similar compounds (44, 47).
Bioremediation
Bio-decontamination is a spontaneous or directed process in which biological (mainly microbiological) processes are used to degrade or transform contaminants into less or non toxic forms, thus mitigating environmental contamination (11). Unquestionably, one of the most important challenges of humanity in the beginning of the XXI century is transforming our production processes into clean and energetically efficient ones (29). In this sense, T. versicolor (L.:Fr.) Pilát has been one of the most thoroughly studied white-rot basidiomycetes, with regards to the transformation of contaminants (40). It has been frequently reported as a powerful biodegrader of a wide range of toxic agents that can be found deposited in soils and water (industrial effluents). Among the most important contaminants we can count recalcitrant and xenobiotic compounds, some of them very resistant to the biodegrading action of native microorganisms, due to the fact that they are chemically synthesized. However, the ligninolytic system of T. versicolor, due to its non specificity, has shown great effectiveness in degrading and mineralizing several of these chemicals such as pesticides, organo-chlorinated compounds, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), dibutyl phthalate (DBP), synthetic dyes, wood preservatives and synthetic polymers, among others (11-13). The mechanism of this biodegrading system is based on the production of free radicals. This mechanism allows the secreted enzymes to be catalytically active on a large spectrum of organic substrates whose enormous structural diversity remarks the important bioremediation potential of these fungi (29).
Regarding biodegradation, MnP and laccase are the most active of these enzymes. They are directly implied in the discoloration of some synthetic dyes like amarant (monoazo dye) and RB5 (diazo dye), through the activity of MnP; and of RBBR (anthraquinone dye), which is degraded by laccase (12, 48). Similarly, the enzymatic activity of immobilized laccases can catalyze the oxidation of anthracene and benzene (through an indirect mechanism that implies the participation of an oxidizing mediator like 2, 2-azino-bis (3-ethylbenzthiazoline-6- sulfonic acid) (ABTS) (31)), and of chlorophenols, including PCPs, the most recalcitrant pentachlorophenols, into less toxic compounds (45).
Oxidoreductases, laccases and peroxidases have a considerable paper pulp bleaching potential. In this regard, the oxidation of pulp and paper industry effluents, probably through the enzymatic action of laccase from T. versicolor, has been demonstrated (46). Said activity could be enhanced in the presence of mediators such as 2, 2-azino-bis (3-ethylbenzthiazoline- 6-sulfonic acid) or 1-hidroxibenzatriazole (HBT), thus totally delignifying both wooden and linen alkaline pulps (43, 46).
Several biosorption studies (11-13, 47, 49) have demonstrated the ability of the mycelium of this fungus to scavenge, capture and accumulate in its biomass certain heavy metals like Cu2+, Pb2+, Zn2+, Cd(II) and Hg(II), which can be found producing remarkable contamination in aquatic or terrestrial environments. Hence, T. versicolor can be said to have high potential as an absorption and biosorption agent for retiring heavy metals in several environments, especially aquatic ones, as is the case of sewage waters. This process depends mainly on pH and metal concentration levels in the medium that is to be cleaned (50, 51).
Potential applications
Recent studies have considered the application of T. versicolor (L.:Fr.) Pilát as a possible biological control for mycotoxin producing fungi (Aspergillus parasiticus) in foods such as corn and wheat. Among the secondary metabolites that these fungi are able to produce, aflatoxins hold an important place, in as much as they are considered to be mutagenic and carcinogenic for humans and animals. Research on this topic has shown that T. versicolor extracts are capable of inhibiting the production of these toxins by 40 to 90% in liquid cultures, as well as in wheat and corn seeds inoculated with the contaminating agent. Said ability of this fungus could be related to the oxidizing effect of its extracts, besides the activity of B-glucans, which are also likely to be involved (21).
CONCLUSIONS
The current review presents T. versicolor (L.: Fr.) Pilát, as a biologically versatile fungus. In consequence, it can be used as the basis of several medicinal, biotechnological and environmental applications. Some of these constitute worldwide applied traditional uses, while some others still need further research, in order to reach adequate industrial developments.
There is sufficient evidence of the effectiveness of T. versicolor as a powerful anti-cancer agent, as well as of its antiviral, antibacterial and analgesic qualities. For this reason, more thorough clinical research should be conducted to confirm the medicinal properties of this mushroom as an immune system supporter for patients diagnosed with chronic diseases such as cancer.
The immunotherapeutic properties of T. versicolor based nutrition result from the supply of (i) complex polysaccharides coupled to proteins that promote the strengthening of the immune system and exhibit antitumor activity; (ii) enzymes that prevent oxidative stress and inhibit cellular growth; and (iii) secondary metabolites involved in several biological processes.
Several research studies have highlighted the ability of this fungus to discolor a variety of synthetic dyes, and to degrade a wide set of toxic contaminants that are present in soils and water. Nonetheless, the mechanism by which all these processes are carried out has not been cleared yet, and is likely to be useful in the design and optimization of large scale processes. T. versicolor (L.:Fr.) Pilát possesses a complex mechanism involving enzymes that attack lignin directly, like for example lignin peroxidase (LiP), Manganese peroxidase (MnP) and Laccase.
T. versicolor (L.: Fr.) Pilát possesses several important enzymes, among which we can count (i) hydrogen peroxide producing enzymes, which aid the catalytic action of peroxidases (for example: glucose oxidase and glyoxal oxidase); (ii) enzymes that are able to participate in the rupture of phenols, aldehydes, other lignin derivatives (such as quinone reductase) or polycyclic aromatic hydrocarbons; (iii) enzymes that are necessary for preventing eventual excessive accumulations of hydrogen peroxide; and (iv) enzymes belonging to the redox cycles of hydroquinones (for example: catalase, superoxide dismutase and glutathione peroxidase).
T. versicolor is certainly a very useful organism for degrading an ample variety of contaminants, as well as for the production of metabolites. It is a biologically versatile fungus which could therefore serve as the basis of various biotechnological and environmental applications. Some of these constitute traditional applications that are currently practiced throughout the world; and some need further research and development. However, regarding the obtention of these products, and seeking to obtain good performances in contaminant degradation, it is chiefly important to optimize the currently available culture mediums with carbon, nutrient and mediating compound sources.
The present review remarks the potential applications of T. versicolor (L.: Fr.) Pilát in different biotechnological fields. Notwithstanding, research is still at the laboratory test stage, and industrial applications are incipient.
REFERENCES
1. Hobbs C. Medicinal Value of Turkey Tail Fungus Trametes versicolor (L.:Fr.) Pilát (Aphyllophoromycetideae). Int J Med Mushrooms. 2004; 6 (3): 24-30. [ Links ]
2. Kozarski M, Klaus A, Niksic M, Vrvic M, Todorovic N, Jakovljevic D, Van Griensven L. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J Food Comp Anal. 2012 Feb 28. Forthcoming. [ Links ]
3. Lin F, Lai Y, Yu H, Chen N, Chang C, et al. Effects of Lycium barbarum extract on production and immunomodulatory activity of the extracellular polysaccharopeptides from submerged fermentation culture of Coriolus versicolor. Food Chem. 2008 Sep 15; 110 (2): 446-453. [ Links ]
4. Orhan I, Ustun O. Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. J Food Comp Anal. 2011 May; 24 (3): 386-390. [ Links ]
5. Harhaji Lj, Mijatovic S, Maksimovic-Ivanic D, Stojanovic I, Momcilovic M., et al. Anti-tumor effect of Coriolus versicolor methanol extract against mouse B16 melanoma cells: In vitro and in vivo study. Food Chem Toxicol. 2008 Jan 28; 46 (5): 1825-1833. [ Links ]
6. Lee C, Yang X, Wan J. The culture duration affects the immunomodulatory and anticancer effect of polysaccharopeptide derived from Coriolus versicolor. Enzyme and Microbial Technology. 2006; (38): 14-21. [ Links ]
7. Cui J, Chisti Y. Polysaccharopeptides of Coriolus versicolor: physiological activity, uses, and production. Biotechnol Adv. 2003 Apr; 21 (2): 109-122. [ Links ]
8. Laua C, Ho C, Kim C, Leung K, Fung K, et al. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004 Jul 2; 75 (7): 797-808. [ Links ]
9. Michaela K, Volker S, Manfred A, Martin F, Doris S. Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour Technol. 2011 Nov 26; (104): 298-304. [ Links ]
10. Hor S, Ahmad M, Farsi E, Lim C, Asmawi M, Yam M. Acute and subchronic oral toxicity of Coriolus versicolor standardized water extract in Sprague-Dawley rats. J Ethnopharmacol. 2011 Jul 8; 137 (3): 1067-1076. [ Links ]
11. Hai I, Modin O, Yamamoto K, Fukush K, Nakajima F, Nghiemd L. Pesticide removal by a mixed culture of bacteria and white-rot fungi. Journal of the Taiwan Institute of Chemical Engineers 2011. Forthcoming 2012. [ Links ]
12. Saratale R, Saratale G, Chang J, Govindwar S. Bacterial decolorization and degradation of azo dyes: A review. Journal of the Taiwan Institute of Chemical Engineers. 2011 Jan; 42 (1): 138- 157. [ Links ]
13. Kaushik P, Malik A. Fungal dye decolourization: Recent advances and future potential. Environ Int. 2009 Jan; 35 (1): 127-141. [ Links ]
14. Subbaiah M, Yuvaraja G, Vijaya Y, Krishnaiah A. Equilibrium, kinetic and thermodynamic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by fungus (Trametes versicolor) biomass. Journal of the Taiwan Institute of Chemical Engineers. 2011 Nov; 42 (6): 965-971. [ Links ]
15. Sahan T, Ceylan H, Sahine N, Aktas N. Optimization of removal conditions of copper ions from aqueous solutions by Trametes versicolor. Bioresour Technol. 2010 Feb 10; 101 (12): 4520-4526. [ Links ]
16. Akar S, Akar T, Kaynak Z, Anilan B, Cabuk A, et al. Removal of copper (II) ions from synthetic solution and real wastewater by the combined action of dried Trametes versicolor cells and montmorillonite. Hydrometallurgy. 2009 Jul 2; 97: 98-104. [ Links ]
17. Torres TMG, Ríos HA, Medina RMA, Barrios AL, Mosquera MH, et al. Distribución de algunos géneros de macromicatos en el municipio de Quibdó. Revista Institucional de la Universidad Tecnológica del Chocó. 2002; 16: 53-56. [ Links ]
18. Torres AP, Córdoba MKY, Torres TMG. Aportes al conocimiento de los macromicetos de la Estación Ambiental de Tutunendo, municipio de Quibdó. Revista Institucional de la Universidad Tecnológica del Chocó. 2003; 18: 10-15. [ Links ]
19. Guzmán G, Torres-Torres MG, Ramírez-Guillén F, Ríos- Hurtado A. Introducción al conocimiento de los macromicetos del Chocó, Colombia. Rev Mex Micol. 2004; (19): 33-43. [ Links ]
20. Li F, Wen H, Liu X, Zhou F, Chen G. Gene cloning and recombinant expression of a novel fungal immunomodulatory protein from Trametes versicolor. Protein Expr Purif. 2012 Apr; 82 (2): 339-344. [ Links ]
21. Zjalic S, Reverberi M, Ricelli A, Granito V, Corrado F, Fabbri A. Trametes versicolor: A possible tool for aflatoxin control. Int J Food Microbiol. 2006 Apr 1; 107 (3): 243-249. [ Links ]
22. Duncan S, Schilling J. Carbohydrate-hydrolyzing enzyme ratios during fungal degradation of woody and non-woody lignocellulose substrates. Enzyme and Microbial Technol. 2010 Dec 8; 47 (7): 363-371. [ Links ]
23. Yang Y, Fuying Ma, Hongbo Yu, Fangfan Fan, Xia Wan, et al. Characterization of a laccase gene from the white-rot fungi Trametes sp. 5930 isolated from Shennongjia Nature Reserve in China and studying on the capability of decolorization of different synthetic dyes. Biochemical Engineering Journal. 2011; (57): 13-22. [ Links ]
24. Decamps K, Joye I, Haltrich D, Nicolas J, Courtin C, Delcour J. Biochemical characteristics of Trametes multicolor pyranose oxidase and Aspergillus niger glucose oxidase and implications for their functionality in wheat flour dough. Food Chem. 2012; 131: 1485-1492. [ Links ]
25. Asgher M, Azim N, Bhatti H. Decolorization of practical textile industry effluents by white rot fungus Coriolus versicolor IBL-04. Biochemical Engineering Journal. 2009 Sep 5; 47 (1-3): 61-65. [ Links ]
26. Harikrishnan R, Kim M, Kim J, Balasundaram C, Heo M. Effect of Coriolus versicolor supplemented diet on innate immune response and disease resistance in kelp grouper Epinephelus bruneus against Listonella anguillarum. Fish Shellfish Immunol. 2012 Feb; 32 (2): 339-344. [ Links ]
27. Chan S, Yeung J. Effects of polysaccharide peptide (PSP) from Coriolus versicoloron the pharmacokinetics of cyclophosphamide in the ratand cytotoxicity in HepG2 cells. Food Chem Toxicol. 2006 May; 44 (5): 689-694. [ Links ]
28. Mycology Research Laboratories. Coriolus- MRL supports the immune system [Internet]. OceanaLab & Versión Two Desing. 2007. [Consultado: 30 de agosto de 2009]. Disponible en: http://www.mycologyresearch.com/products.asp?product=Coriolus. [ Links ]
29. Dávila G, Vázquez-Duhalt R. Enzimas ligninolíticas fúngicas para fines ambientales. Mensaje Bioquím. 2006; 30 (1): 29- 55. [ Links ]
30. Ragupathi G, Yeung K , Leung P, Lee M, Lau C, et al. Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine. 2008 Sep 2; 26 (37): 4860-4865. [ Links ]
31. Rodríguez-Rodríguez C, García-Galán M, Blánquez P, Díaz- Cruz S, Barceló D, et al. Continuous degradation of a mixture of sulfonamides by Trametes versicolor and identification of metabolites from sulfapyridine and sulfathiazole. J Hazar Mater. 2012 Apr 30; 213-214: 347-354. [ Links ]
32. Prévoteau A, Faure C. Effect of onion-type multilamellar liposomes on Trametes versicolor laccase activity and stability. Biochimie. 2012 Jan; 94 (1): 59-65. [ Links ]
33. Gil D, Rebelo M. Gallic acid interference on polyphenolic amperometric biosensing using Trametes versicolor laccase. J Molecular Catalysis B: Enzymatic. 2011 Jun 16; 72: 193-198. [ Links ]
34. Moreno C, González A, Blanco M. Tratamientos biológicos de suelos contaminados: contaminación por hidrocarburos. Aplicaciones de hongos en tratamientos de biorrecuperación. Rev Iberoam Micol. 2004; 21 (1): 103-120. [ Links ]
35. Masud S k, Anantharaman N. Activity enhancement of ligninolytic enzymes of Trametes versicolor with bagasse powde. Afr J of Biotechnol. 2006 Jan 16; 5 (1): 189-194. [ Links ]
36. Martín C, González A, Blanco MJ. Tratamientos biológicos de suelos contaminados: contaminación por hidrocarburos. Aplicaciones de hongos en tratamientos de biorrecuperación. Rev Iberoam Micol. 2004; 21 (1): 103-120. [ Links ]
37. Jang M, Ryu W, Cho M. Enhanced production of laccase from Trametes sp. by combination of various inducers. Biotechnol Bioprocess Eng. 2006; 11 Lorenzo M, Moldes D, Rodríguez S, Sanromán M. Inhibition of lacc ase activity from Trametes versicolor by heavy metals and organic compounds. Chemosphere. 2005; 60 (8): 1124-1128. [ Links ]
38. Dodor D, Hwang H, Ekunwe S. Oxidation of anthr acene and benzo[a]pyrene by immobilized laccase from Trametes versicolor. Enzyme Microbial Technol. 2004; 35 (2-3): 210-217. [ Links ]
39. Camarero S, Garcia O, et al. Efficient bleachin g of non-wood high-quality paperpulp using laccase-mediator system. Enzyme Microbial Technol. 2004; 35 (2-3): 113-120. [ Links ]
40. Kasikara N, Sariisik M, Telefoncu A. Laccase: productio n by Trametes versicolor and application to denim washing. Process Biochem. 2005; 40 (5): 1673-1678. [ Links ]
41. Kolb M, Sieber V, Amann M, Faulstich M, Schieder D. Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour Technol. 2012 Jan; 104: 298-304. [ Links ]
42. Valášková V, Baldrian P. Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Res Microbiol. 2006; 157 (2): 119-124.
43. Novotny C, Svobodova K, et al. Ligninolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biol Biochem. 2004; 36 (10): 1545-1551. [ Links ]
44. Lee S, Koo B, et al. Biodegradation of dib utylphthalate by white rot fungi and evaluation on its estrogenic activity. Enzyme Microbial Technol. 2004; 35 (5): 417-423. [ Links ]
45. Gómez-Dorado C, Martínez-Salgado M, et al. Estudio del efecto de dos inductores y un protector enzimático sobre la actividad de las enzimas Mn-peroxidasa y lacasa producidas por Trametes versicolor y su efecto en la decoloración de efluentes de la industria papelera. Univ Sci. 2005; 10 (2): 37-45. [ Links ]
46. Badia-Fabregat M, Rodríguez-Rodríguez C, Gago-Ferrero P, Olivares A, Piña B, et al. Degradation of UV flters in sewage sludge and 4-MBC in liquid medium by the ligninolytic fungus Trametes versicolor. J Environ Manage. 2012 Apr 7; 104C: 114-120. [ Links ]
47. Garmaroody E, Resalati H, Fardimc P. Modification of kraft pulp fibers by fungal pre-treatment of Iranian hornbeam chips. International Biodeterioration & Biodegradation 2012 May; 70: 20-26. [ Links ]
48. Cabana H, Ahamed A, Leduc R. Conjugation of laccase from the white rot fungus Trametes versicolor to chitosan and its utilization for the elimination of triclosan. Bioresour Technol. 2011 Jan; 102 (2): 1656-1662. [ Links ]
49. Jelic A, Cruz-Morato C, Marco-Urrea E, Sarra M, Perez S, et al. Degradation of carbamazepine by Trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water research. 2012 Mar 15; 46 (4): 955-964. [ Links ]
50. García-Galán M, Rodríguez-Rodríguez C, Vicent T, Caminal G, Díaz-Cruz M, Barceló D. Biodegradation of sulfamethazine by Trametes versicolor: Removal from sewage sludge and identification of intermediate products by UPLC-QqTOF-MS. Sci Total Environ. 2011 Nov 15; 409 (24): 5505-5512. [ Links ]
51. García-Galán M, Rodríguez-Rodríguez C, Vicent T, Caminal G, Díaz-Cruz M, Barceló D. Biodegradation of sulfamethazine by Trametes versicolor: Removal from sewage sludge and identification of intermediate products by UPLC-QqTOF-MS. Sci Total Environ. 2011 Nov 15; 409 (24): 5505-5512. [ Links ]