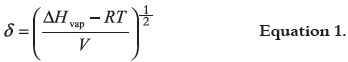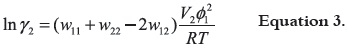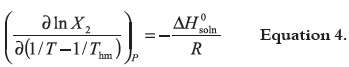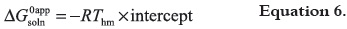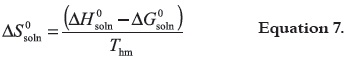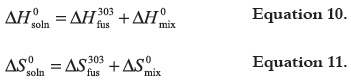Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.19 no.1 Medellín Jan./Apr. 2012
PHARMACEUTICAL INDUSTRY
SOLUTION THERMODYNAMICS OF TRICLOSAN AND TRICLOCARBAN IN SOME VOLATILE ORGANIC SOLVENTS
TERMODINÁMICA DE DISOLUCIÓN DE TRICLOSÁN Y TRICLOCARBÁN EN ALGUNOS SOLVENTES ORGÁNICOS VOLÁTILES
Daniel R. DELGADO1; Andres R. HOLGUIN1; Fleming MARTÍNEZ1*
1 Grupo de Investigaciones Farmacéutico-Fisicoquímicas. Departamento de Farmacia, Facultad de Ciencias. Universidad Nacional de Colombia. A.A. 14490. Bogotá D.C. Colombia.
* Corresponding author: fmartinezr@unal.edu.co.
Received: 18 January 2011
Accepted: 31 January 2012
ABSTRACT
Thermodynamic functions of Gibbs energy, enthalpy, and entropy for the solution processes of the antimicrobial drugs Triclosan and Triclocarban in five volatile organic solvents were calculated from solubility values at temperatures from 293.15 to 313.15 K. Triclosan and Triclocarban solubility was determined in acetone, acetonitrile (AcCN), ethyl acetate (AcOEt), methanol (MetOH), and cyclohexane (CH). The excess of Gibbs energy and the activity coefficients of the solutes were also calculated. The Triclosan solubilities were greater than those of Triclocarban in all the solvents studied. At 298.15 K the solubility diminished for Triclosan in the order, acetone > AcOEt > AcCN > MetOH > CH, while it diminished for Triclocarban in the order, acetone > AcOEt > MetOH > AcCN > CH. On the other hand, thermodynamic quantities relative to the transfer process of these drugs from CH to all other organic solvents, as well as from water to organic solvents for Triclosan were also calculated in order to estimate the hydrogen-bonding contributions.
Keywords: Antimicrobial drugs, Solubility, Transfer, Chemical thermodynamics.
RESUMEN
En esta investigación se calcularon las funciones termodinámicas: energía de Gibbs, entalpía y entropía para los proceses de disolución de los agentes antimicrobianos Triclosán y Triclocarbán a partir de los valores de solubilidad a diferentes temperaturas entre 293,15 y 313,15 K en cinco solventes orgánicos volátiles. La solubilidad de los dos agentes se determinó en acetona, acetonitrilo (AcCN), acetato de etilo (AcOEt), metanol (MetOH), y ciclohexano (CH). También se calcularon las energías Gibbs de exceso y los coeficientes de actividad de los solutos. Las solubilidades de Triclosán fueron mayores que las de Triclocarbán en todos los solventes estudiados. A 298,15 K la solubilidad de Triclosán disminuyó en el orden, acetona > AcOEt > AcCN > MetOH > CH, mientras que en caso de Triclocarbán, disminuyó en el orden, acetona > AcOEt > MetOH > AcCN > CH. De otro lado, también se calcularon las cantidades termodinámicas relativas a los procesos de transferencia de estos dos fármacos desde CH hasta los otros solventes orgánicos, así como las de la transferencia de Triclosán desde el agua hasta los solventes orgánicos, con el fin de hacer estimaciones de las contribuciones por enlaces de hidrógeno.
Palabras clave: Fármacos antimicrobianos, Solubilidad, Transferencia, Termodinámica química.
INTRODUCTION
Triclosan (TS, 5-chloro-2-(2,4-dichlorophenoxi)- phenol, its molecular structure is showed in Table 1), is a potent synthetic bactericide and fungicide agent with notable chemical stability and persistent activity (1). Due to these features, TS has been extensively used along the years in a diversity of topical applications (2). In 1997 it was approved by the FDA for use in oral care products such as toothpastes, and its application gained even more impact with the development of mouthwashes and other formulations for plaque prevention and control of periodontal disease (3-5). In 2003, Ethicon Inc. has introduced poly(lactic-glycolic acid) biodegradable surgical sutures coated with TS (Vicryl® Plus) (6).
One important limitation in the development of TS-loaded topical products is the poor aqueous solubility of the drug (7). This behavior stems from its high hydrophobicity. On the other hand, the presence of one aromatic –OH group, that is ionizable at pH > 10, enables better solubilization under alkaline pH conditions. However, such alkalinity is incompatible with the vast majority of pharmaceutical applications. Several approaches were investigated in order to improve the apparent solubility of TS in neutral aqueous medium. Some investigators designed complexes with β-cyclodextrins and studied the effect of ionization and polymers nature on the formation of aggregates and higher-order complexes (8-10). Grove et al., 2003 (11) investigated other molecular complexes, micelles and the in situ formation of organic salt; findings showed that the solubilizers studied increased the solubility of TS from 80- to 6000-fold; furthermore, although the bacteriostatic efficacy of TS was significantly increased when solubilized with N-methylglucamine, L-arginine, and ethanolamine, increased solubilization did not increase the effectiveness of TS for all solubilizers tested (11). Maestrelli et al., 2006 (12) developed chitosan-hydroxypropyl cyclodextrin nanocarriers and investigated the water-solubilization of TS; findings showed a 20- fold increase in the solubility of the drug (12). In another work, Steinberg et al., 2006 (13) reported the development of ethylcellulose TS-containing buccal patches for sustained release of the drug; the device effectively released TS following a Higuchi model and affected the viability of Streptococcus mutans, a frequent pathogen in periodontal disease (13). More recently, the solubilization of TS by means of inclusion into poloxamine (a four-arms poly(ethylene oxide)-poly(propylene oxide) block copolymer) polymeric micelles in a broad range of pH values and polymer concentrations has been studied (14). Apparent solubility values increase up to 4 orders of magnitude. Moreover, the hydrogen bonding ability played a central role in the drugnanocarrier interaction. Thus, ionized TS (at pH ~12) displayed a weaker affinity for the micelle and this phenomenon rendered lower solubilization extents when compared to lower pH values (14). More importantly, TS-loaded systems showed antibacterial activity in vitro against a broad spectrum of pathogens.
On the other hand, triclocarban (TCC, 3,4,4'-trichlorocarbanilide, its molecular structure is showed in Table 1) is also a synthetic antibacterial agent used as antiseptic in cosmetic and healthconsumer products (15, 16). It presents low acute and chronic toxicity. Due to its molecular nature, TCC remains unionized in a broad range of pH values (pKa = 12.7). Contrary to TS, reports indicated that TCC displays a more limited activity (17, 18). The extremely low solubility of TCC in water (~50 ng mL–1) constrains the development of water-based antibacterial TCC-containing formulations. As aforementioned, different technological approaches are being investigated in order to enhance the solubility of poorly-water soluble drugs (19). For example, the improved aqueous solubility of TCC by means of encapsulation into two branched poly(ethylene oxide)-poly(propylene oxide) (PEO-PPO) block copolymers (poloxamine, Tetronic®) was reported (20).
Besides, it has been well described that the profuse use of these agents has raised important environmental concerns due to its accumulation in wastewater streams (21, 22). As in the case of pre-formulation and formulation process of pharmaceutical dosage forms, toxicity to aquatic life and appearance in drinking water is directly related to its solubility in water. For these reasons, the fundamental aspects of the aqueous dissolution and transfer processes of TS from water to organic solvents with different hydrogen capability used for Quantitative Structure-Activity Relationships (QSAR) studies (namely, octanol, chloroform, isopropyl myristate, and cyclohexane) have been reported (23, 24), whereas for TCC just the thermodynamic quantities of dissolution in some QSAR-relevant solvents have been reported (25). Nevertheless, the information about solubility and solution thermodynamics of these drugs in volatile organic solvents is too scarce.
In this context, the present work studied the solution thermodynamics of TS and TCC in five volatile organic solvents widely used in microencapsulation processes (26). Therefore, the main goal of the present research was to present a more complete and systematic insight on the properties of dissolution and transfer for these drugs. Hence, TS and TCC solubility in acetone, ethyl acetate (AcOEt), acetonitrile (AcCN), methanol (MetOH) and cyclohexane (CH) was determined at temperatures ranging between 293.15 and 313.15 K. From these solubility values, the thermodynamic quantities of solution were calculated by means of the van't Hoff and Gibbs equations for both drugs. In addition, the thermodynamic quantities relatives to the transfer process of these drugs from CH to all the other volatile organic solvents were also calculated in order to estimate the hydrogen-bonding contributions. Moreover, the thermodynamic quantities of TS transfer from water to the same organic solvents were also calculated based on the aqueous solution thermodynamic values reported in the literature (24).
MATERIALS AND METHODS
Reagents
Triclosan USP (TS) was a kind of gift from Ciba C.S. (27); Triclocarban (TCC) A.R. Sigma; acetone A.R. Merck; ethyl acetate (AcOEt) A.R. Merck; Acetonitrile (AcCN) A.R. Merck; methanol (MetOH) A.R. Merck; cyclohexane (CH) A.R. Merck; absolute ethanol (EtOH) A.R. Merck. All reagents were used without further purification.
Solubility determinations
An excess of TS or TCC was added to 20 cm3 of each organic solvent evaluated in sealed dark glass flasks. The solid–liquid mixtures were then allowed with stirring in a thermostatic mechanical shaker (Julabo SW23) kept at 313.15 ± 0.05 K for at least five days to reach the equilibrium (this equilibrium time was established by quantifying the drug concentration up to obtain a constant value). Once at equilibrium, supernatant solutions were filtered at isothermal conditions (Millipore Corp. Millex®-13mm filters) in order to remove insoluble particles before analysis. TS concentrations were determined by mass balance, weighting a specific quantity of the respective saturated solution and allowing the solvent evaporation up to constant mass. TCC concentrations were determined by measuring absorbance after appropriate dilution with absolute ethanol and interpolation from previously constructed UV spectrophotometry calibration curve (UV/VIS BioMate 3 Thermo Electron Company). After these procedures, the temperature was decreased in 5.0 K, so stabilizing it in 308.15 K during at least two days, allowing the precipitation of the drug dissolved in excess and quantifying the drug concentration in equilibrium. This procedure was repeated decreasing temperature in 5.0 K up to reach 293.15 K. In order to allow the conversion between concentration scales, the density of the saturated solutions was determined with a digital density meter (DMA 45 Anton Paar, precision ± 0.0001 g cm-3). All experiments were made at least three times and averaged.
RESULTS AND DISCUSSION
Molecular structures of TS and TCC (28) and their most relevant physicochemical properties in solid state are summarized in Table 1 (20, 29). Both drugs act in solution mainly as a Lewis acid (because of the phenolic –OH group in TS and the >N–H groups in TCC) in order to establish hydrogen bonds with proton-acceptor functional groups present in the solvents (i.e. –OH, –O–, and >C=O, and –CN groups), although they also could act as Lewis base because of the oxygen atoms in TS and the carbonyl moiety in TCC.
Experimental solubility of TS and TCC
Table 2 summarizes the experimental solubilities of TCC, expressed in molarity and mole fraction, in addition to the ideal solubilities (X2id) taken from the literature (23, 25). In almost all cases the coefficients of variation for the experimental solubility were smaller than 1.0%.
It can be observed that the highest solubility values in mole fraction for TS and TCC were in acetone at 313.15 K, while the lowest values were found in CH at 293.15 K. In all cases the TS solubilities are almost 100 fold times greater than the TCC ones. This behavior is similar to that found with QSAR solvents (23, 25). On the other hand, at 298.15 K for TS the diminishing order obtained was: acetone > AcOEt > AcCN > MetOH > CH and for TCC it was: acetone > AcOEt > MetOH > AcCN > CH. However, no reports on solubility values for these drugs in the solvents studied are available, and therefore none direct comparison is possible. Experimental solubilities exhibited by TS in acetone and AcOEt as well as by TCC in acetone are greater than the ideal ones at all temperatures studied.
TS and TCC solubility analysis in terms of Hildebrand solubility parameters
Although experimental solubility is a complex phenomenon, the attempts have been proposed in order to explain this important physicochemical property of drugs. One of them was proposed by Hildebrand et al., 1970 (30) in terms of the solubility parameter δ, which is defined as the root square of cohesive energy density, and it is calculated according to equation 1:
where, ΔHvap is the vaporization enthalpy and V is the molar volume. Hildebrand solubility parameters were initially proposed for nonpolar compounds interacting among them by dispersion forces (London forces); nevertheless, TS, TCC and almost all the volatile solvents investigated interact by London forces and also by other more energetic forces, namely, dipolar forces and hydrogenbonding. In this context, Hansen split the general δ values in three partial parameters considering the respective contributions by dispersive forces δd, dipolar forces δp, and hydrogen-bonding δh (31). These subparameters are related to total solubility parameter δT, according to:
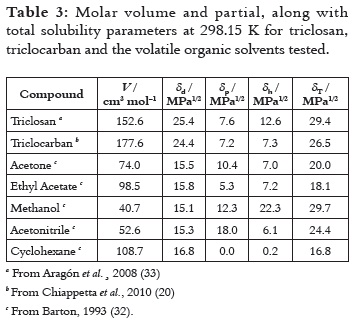
The experimental determination of partial solubility parameters of drugs is not an easy matter, and therefore some calculus methods based on the contribution of groups have been described. The methods more used are those proposed by Fedors and van Krevelen (32). In this context, Table 3 summarizes the TS and TCC solubility parameters reported in the literature (20, 33), where it can be seen that the London forces are the most relevant for these drugs, which could be attributed mainly to their aromatic moieties. In this way, based on the δT values for TS and TCC (29.4 and 26.5 MPa1/2, respectively), these drugs could be considered as semipolar compounds. On the other hand, according to Martin and Bustamante (34), the greatest drug solubility value should be found in solvents with similar δ values. For this reason, Table 3 also summarizes the δ values for the organic solvents tested (32, 35).
Apparently, no similarity in all δ values is observed by comparing TS and TCC in all the volatile solvents tested (as shown in Table 3) when they are related to the equilibrium solubilities (as shown in Table 2). This fact demonstrates that the solubility of a certain drug compound is a more complex phenomenon than that exclusively described by solubility parameters and without considering other properties. In the same way, Figures 1 and 2 clearly show that no simple relation between TS and TCC equilibrium solubilities and the solvents δT values is found.
TS and TCC activity coefficients
The solute activity coefficient in the solution (γ2) is calculated as (X2id / X2) and it is an indication of the deviation presented by both drugs with respect to their ideal behaviors (36). Table 4 summarizes the activity coefficients as a function of temperature. The γ2 of TS in acetone and AcOEt, as well as of TCC in acetone, are lower than unit at all temperatures studied. This finding is because of the experimental solubilities that are greater than the ideal ones. In all cases, γ2 values tend to unit when temperature rises, so being more ideally the solution process for both drugs despite if γ2 is greater or lower than unit. These γ2 diminishing rates are the greatest for both drugs in CH and more specially for TS.
From the different magnitudes obtained for the γ2 values presented in Table 4 an approximate estimation of the respective solute-solvent intermolecular interactions can be made by considering the following expression:
where w11, w22 y w12 represent the solvent-solvent, solute-solute and solvent-solute interaction energies, respectively; V2 is the molar volume of the supercooled liquid solute, and finally, φ1 is the volume fraction of the solvent. In a first approach, the term (V2 φ12 / RT)T,P may be considered approximately constant at the same temperature, and then γ2 depends almost exclusively on w11, w22 and w12 (37). While the term w12 favors the solution process, both w11 and w22 terms are unfavorable for solubility. The contribution of w22 represents the work necessary to transfer drug molecules from the solid to the vapor state; consequently, it could be considered as constant in all the volatile organic solvents studied.
The γ2 values of TS in all the solvents are close to unit, except in CH at low temperatures (close to 7 at 298.15 K). Thanks to that, all the solvents have low polarity (low w11) and the solute is the same in the five cases (with low w22 based on properties of fusion), it is also possible to assume the presence of high interactions w12, except for CH at low temperatures. The results are more disperse for TCC because γ2 values close to unit were found only for acetone and AcOEt. On the other hand, for MetOH and AcCN the γ2 were close to 5 or 10 indicating lower solvent-solute interactions as compared with TS. Finally, for TCC in CH is clear that the term w12 should be too low in order to obtain γ2 values near to ten-thousand.
Apparent thermodynamic functions of solution of TS and TCC
According to van't Hoff analysis modified by Krug et al., 1976 (38), the apparent standard enthalpy change of solution is obtained from the slope of ln X2 vs. 1/T – 1/Thm plot (39) in according to the expression:
where Thm is the mean harmonic temperature, which is calculated as:
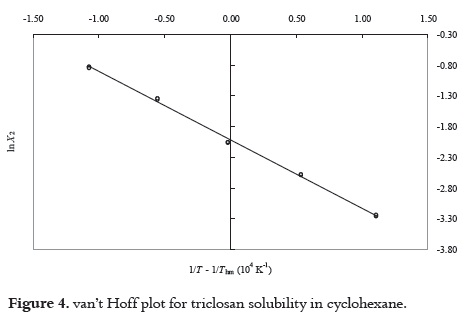
where n is the number of temperatures studied (38, 39). In the present case the Thm value obtained is just 303 K. The modified van't Hoff plots for TS solubility in MetOH, AcOEt, AcCN and CH are presented in the Figure 3 while its behavior in CH is presented in the Figure 4 (it was necessary to make another plot because of the great effect of temperature on the TS solubility in this aprotic solvent). Likewise, Figure 5 shows the behavior of TCC in all the solvents. Linear regression models with good statistical parameters were obtained for both drugs in all the organic solvents studied.
The apparent standard Gibbs energy change for the solution process (ΔG0appsoln), considering the approach proposed by Krug et al. 1976 (38), is calculated by means of:
in which, the intercept used is the one obtained in the analysis by treatment of ln X2 as a function of 1/T – 1/Thm. Finally, the standard entropic change for solution process (ΔS0soln) is obtained from the respective ΔH0soln and ΔG0soln values by using:
Table 5 summarizes the apparent standard thermodynamic functions for the experimental solution processes of TS and TCC in all the organic solvents investigated, including those functions reported for the ideal processes (23, 25). In order to calculate the thermodynamic magnitudes of experimental solution, some methods to calculate the propagation of uncertainties were used (40). It is found that the standard Gibbs energy of solution is positive in all cases; i.e., the solution process apparently is not spontaneous, which may be explained in terms of the concentration scale used (mole fraction), where the reference state is the ideal solution having the unit as concentration of both drugs (the solid pure solute). As expected, in all cases solution enthalpies are positive.
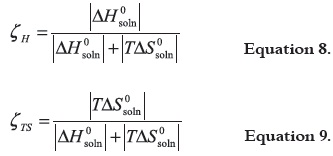
With the aim to compare the relative contributions by enthalpy (ζH) and entropy (ζTS) toward the solution process, equations 8 and 9 were employed respectively (41):
From the values shown in Table 5, it follows that the contributions for the experimental solution processes of TS in all solvents are very similar to those obtained for the ideal process with low enthalpy predominating (ζH near to 0.52). Oppositely, for TCC the experimental findings are more disperse compared with TS and the enthalpy contributes in greater amount (ζTS near to 0.70).
Apparent thermodynamic functions of mixing of TS and TCC
The solution process may be represented by the following hypothetical stages (36):
where, the respective partial processes toward the drug dissolution are solute fusion and mixing at the same temperature (303 K), which permits to calculate the partial thermodynamic contributions to overall solution process by means of equations 10 and 11, respectively.
where, ΔH303fus and ΔS303fus represent the thermodynamic functions of the fusion process at harmonic temperature (303 K). Nevertheless, for the reasons described in the literature (23-25), in the following analysis the ΔH0idsoln and ΔS0idsoln values (for ideal solution processes) were used instead of ΔH303fus and ΔS303fus This statement has been used previously with several other drugs in similar volatile solvents (42, 43). Thermodynamic functions of mixing of TS and TCC are summarized in Table 6.
The partial contributions by ideal solution (related to solute fusion process) and mixing processes to the enthalpy and entropy of drug solution, show that ΔH0idsoln and ΔS0idsoln are positive (as shown in Table 5), while the contribution of the thermodynamic functions relative to mixing process toward the solution process is variable according to each drug. For TS, ΔG0mix , ΔH0mix and ΔS0mix are negative in acetone and AcOEt and positive in all the other solvents, whereas for TCC these quantities are negative just for acetone and positive in all the other solvents, except for MetOH where the entropy of mixing is negative. It can be concluded that the solution process of TS in acetone and AcOEt, and TCC in acetone are driven mainly by the enthalpy of mixing (because of the negative values), whereas for both drugs in all the other solvents the solution process is driven by the entropy of mixing (because of the positive values), except for TCC in MetOH where nor enthalpy or entropy driving is found.
It is well known that the net variation in ΔH0mix values results from the contribution of several types of interactions. The enthalpy of cavity formation (required for solute accommodation) is endothermic because energy must be supplied against the cohesive forces of the solvent. This process decreases the solubility. On the other hand, the enthalpy of solute-solvent interaction is exothermic and results mainly from van der Waals and Lewis acid-base interactions.
The particular high values obtained for the thermodynamic functions of mixing in CH for both drugs imply that a great quantity of energy is required to overcome the CH-CH London forces present in this aprotic solvent which is not compensated later on solvent-solute interactions. Otherwise, the negative values obtained in enthalpy and entropy of mixing for TS in acetone and AcOEt and for TCC in acetone could indicate that the hydrogen bonds established between both drugs and these solvents are so greater than the acetone-acetone and AcOEt- AcOEt intermolecular interactions which lead to energy release upon the mixing process.
The mixing behaviors obtained in AcCN are so different for both drugs, being close to ideality for TS but clearly non ideal for TCC. This result could be interpreted by considering that TS is more acidic than TCC and thus the solvent-solute interactions by hydrogen bonding are bigger for the first drug, because of its –OH group.
TS and TCC apparent thermodynamic quantities of transfer from CH to the other volatile organic solvents
In order to contribute with the generation and systematization of thermodynamic quantities of transfer useful in QSAR studies, these values were calculated for the transfer of TS and TCC from CH to the other volatile organic solvents.
Gibbs energy, enthalpy and entropy of transfer for both drugs, including the respective ζH and ζTS values, are shown in Table 7. These thermodynamic quantities were calculated as the differences between the solution functions in the other organic solvents (as shown in Table 5) and those for CH presented in the same Table. According to the values shown in Table 7, the transfer process of both drugs from CH to all other organic solvents is spontaneous (ΔG0CH->org< 0) and it is driven by enthalpy (ΔG0CH->org< 0 and ΔS0CH->org< 0), except for TCC from CH to AcCN, where the process is driven by entropy (ΔH0CH->org> 0 and ΔS0CH->org> 0). On the other hand, the contributions by enthalpy and entropy to the transference are hence similar for TS, and it is very interesting to note that the respective magnitudes are the same for all the solvents. Oppositely, in the case of TCC the enthalpy is the main contributor to transfer process in all cases (ζH > 0.62).
As has been described earlier (23, 25), in the net drug transfer process between hydrocarbons and organic solvents with hydrogen-bonding capability as donors or acceptors, the enthalpic and entropic changes imply, respectively, the energetic requirements and the molecular randomness (increase or decrease in the molecular disorder). Broadly speaking, the behavior presented in each phase should be considered independently, before and after the partitioning process.
Since hypothetically the solute is initially present only in the aprotic hydrocarbon phase, the generation of a cavity in the hydrogen-bonding organic medium intended to accommodate the solute after the transfer process is required. This is an endothermic phenomenon, since an energy supply is necessary to overcome the solvent-solvent interaction of the hydrogen-bonded organic solvent molecules. When the solute molecules are accommodated in the organic phase an amount of energy is released, mainly due to formation of new hydrogen bonds between the molecules of the drug and the solvent.
On the other hand, after this, a certain number of solute molecules have migrated from the hydrocarbon solvent to the organic phase, up to reach the hypothetical equilibrium, so the original cavities occupied by the drug in the hydrocarbon phase have been now occupied by CH molecules. This event produces an energy release due to CH-CH London interactions. Thus, the negative enthalpy values of transfer obtained could be explained as the strong solute-solvent interactions due to hydrogenbonding between TS or TCC and the organic solvents, which further diminishes the entropy by drug immobilization inside the solvents.
TS apparent thermodynamic quantities of transfer from aqueous media to the volatile organic solvents
As in the case of pre-formulation and formulation process of pharmaceutical preparations, toxicity to aquatic life and appearance of this drug in drinking water is directly related to solubility in water. In order to present more complete thermodynamic information about transfer properties of TS, Table 8 shows the thermodynamic quantities of transfer for this drug from water to the volatile organic solvents studied. In similar way to that made with the drugs transfer from CH to the other organic solvents, it is important to illustrate the hypothetical events present in the process of transfer of TS from water to organic solvents. Besides Gibbs energy, the enthalpic and entropic changes associated are also important, and imply the energetic requirements and the molecular randomness (increase or decrease in the molecular disorder) involved in the net transfer, respectively.
According to the values shown in Table 8, it follows that the Gibbs energy of transfer is negative in all cases indicating the preference of TS for organic media. On the other hand, except for CH, the enthalpies and entropies of transfer are negative and positive respectively, indicating both enthalpy and entropy driving on the transfer processes. Oppositely, for the TS transfer to CH, just entropy driven is found. With respect to the respective contributions by enthalpy and entropy toward the transfer processes it is found that the energetic term predominates for the TS transfer to acetone and AcOEt, whereas the organizational term predominates in the case of the other organic solvents.
In general terms, the behavior presented in each phase, before and after the transfer process, should be considered independently. Since initially the solute is present in water, then, it is necessary to create a cavity in the organic medium in order to accommodate the solute after the transfer process. This is an endothermic event, since an energy supply is necessary to separate the organic solvent molecules. When the solute molecules are accommodated within the organic phase, an amount of energy is released due to the generation of solute-organic solvent interactions. This general event implies an entropy increase in this organic medium due to the liquid-liquid and solute-solvent mixing process. On the other hand, the original cavities occupied by the drug in the aqueous phase have been now occupied by water molecules; this phenomenon takes place after a certain number of solute molecules have migrated from the aqueous to the organic phase, until reaching the equilibrium. This event produces an energy release due to the formation of water-water interactions.
However, depending on the nonpolar groups present in the molecular structure of the drug, it is also necessary to consider the possible disruption of the water-structure around the drug, namely, the water molecules organized by hydrogen bonding around the alkyl or aromatic groups (effect known as hydrophobic hydration). This event in particular implies an intake of energy, in addition to a local entropy increased by the separation of some water molecules which originally were associated among them by hydrogen bond (44). This is probably the main reason of the high thermodynamic quantities obtained in the hypothetical transfer of TS from water to CH.
Nevertheless, despite the treatment of transfer made here from solubilities, it would be very important to calculate the previously discussed thermodynamic quantities of transfer, but obtained from partition coefficients in those non water-miscible solvents (AcOEt and CH), and confronting them against the apparent values presented in Table 8.
It is also necessary to considerate that the partitioning experiments are carried out at low drug concentrations where the solute-solute interactions are not present (45); whereas, in the solubility analysis these interactions would be present in some organic solvents because of the high solubility values in several cases, and therefore, the thermodynamic quantities obtained also include these interactions, in addition to the solute-solvent ones.
Moreover, in order to identify and understand the specific interactions presented between TS and the aqueous and organic solvents studied, it would be very important to dispose information about UV, IR and NMR spectral data, as well as to DSC and dissolution calorimetric values, among others.
CONCLUSIONS
Based on all topics discussed previously, it is clear that the solubility and solution thermodynamics of both drugs is not simple as expected because of the non polar nature of the majority of solvents studied. In contrast, the differences between both drugs in acidic behaviors, molar volumes, and polarities as described by Hildebrand solubility parameters, really affect these physicochemical properties. Ultimately, it can be said that the solubility data presented in this report amply the physicochemical information about these antimicrobial drugs.
ACKNOWLEDGMENTS
We want to thank Ciba C.S. and Prof. Alejandro Sosnik from the University of Buenos Aires, for donating us the TS and TCC samples studied and also to the DIB-DINAIN of the National University of Colombia (NUC) for the financial support. Additionally, we would like to thank the Department of Pharmacy of NUC for facilitating the equipment and laboratories used.
REFERENCES
1. Bhargava HN, Leonard PA. Triclosan: Applications and safety. Am J Infect Control. 1996 Jun; 24 (3): 209-218. [ Links ]
2. Jones RD, Jampani HB, Newman JL, Lee AS. Triclosan: A review of effectiveness and safety in health care settings. Am J Infect Control. 2000 Apr; 28 (2): 184-196. [ Links ]
3. Food and Drug Administration. FDA approves first toothpaste for gum disease, FDA Talk Paper; 1997 Jul 14. [ Links ]
4. Barnett ML. The role of therapeutic antimicrobial mouthrinses in clinical practice: Control of supragingival plaque and gingivitis. J Am Dental Assoc. 2003 Jun; 134 (6): 699-704. [ Links ]
5. Hioe KPKJ, van der Weijden GA. The effectiveness of selfperformed mechanical plaque control with triclosan containing dentifrices. Int J Dent Hyg. 2005 Nov; 3 (4): 192-204. [ Links ]
6. Storch M, Scalzo H, Van-Lue S, Jacinto G. Physical and functional comparison of coated Vicryl Plus antibacterial suture (coated polyglactin 910 suture with tricloscan) with Vicryl suture (coated polyglactin 910 suture). Surg Infect (Larchmt). 2002; 3 (Suppl 1): S65-S77. [ Links ]
7. Loftsson T, Leeves N, Bjornsdottir B, Duffy L, Masson M. Effect of cyclodextrins and polymers on triclosan availability and substantivity in toothpastes in vivo. J Pharm Sci. 1999 Dec; 88 (12): 1254-1258. [ Links ]
8. Lu J, Hill MA, Hood M, Greeson Jr DF, Horton JR, Orndoff PE, et al. Formation of antibiotic, biodegradable polymers by processing with Irgasan DP300R (Triclosan) and its inclusion compound with β-cyclodextrin. J Appl Polym Sci. 2001 Oct 10; 82 (2): 300-309.
9. Loftsson T, Össurardótti ÍB, Thorsteinsson T, Duan M, Másson M. Cyclodextrin solubilization of the antibacterial agents triclosan and triclocarban: Effect of ionization and polymers. J Incl Phenom Macro. 2005 Jun; 52 (1-2): 109-117. [ Links ]
10. Duan MS, Zhao N, Össurardóttir ÍB, Thorsteinsson T, Loftsson T. Cyclodextrin solubilization of the antibacterial agents triclosan and triclocarban: Formation of aggregates and higher-order complexes. Int J Pharm. 2005 Jun 13; 297 (1-2): 213-222. [ Links ]
11. Grove C, Liebenberg W, Du Preez JL, Yang W, De Villiers MM. Improving the aqueous solubility of triclosan by solubilization, complexation, and in situ salt formation. J Cosmet Sci. 2003 Nov-Dec; 54 (6): 537-550. [ Links ]
12. Maestrelli F, García-Fuentes M, Mura P, Alonso MJ. A new drug nanocarrier of chitosan and hydroxypropylcyclodextrin. Eur J Pharm Biopharm. 2006 Jun; 63 (2): 79-86. [ Links ]
13. Steinberg D, Tal T, Friedman M. Sustained-release delivery systems of triclosan for treatment of Streptococcus mutans biofilm. J Biomed Mater Res B Appl Biomater. 2006 May; 77 (2): 282-286. [ Links ]
14. Chiappetta DA, Degrossi J, Teves S, D'Aquino M, Bregni C, Sosnik A. Triclosan-loaded poloxamine micelles for enhanced antibacterial activity against biofilm. Eur J Pharm Biopharm. 2008 Jun; 69 (2): 535-545. [ Links ]
15. Breneman DL, Hanifin JM, Berge CA, Kewick BH, Neumann PB. The effect of antibacterial soap with 1.5% triclocarban on Staphylococcus aureus in patients with atopic dermatitis. Cutis. 2000 Oct; 66 (4): 296-300. [ Links ]
16. Luby S, Agboatwalla M, Feikin D, Painter J, Billhimer W, Altaf A, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005 Jul 16; 366 (9481): 225-233. [ Links ]
17. Beaver DJ, Roman DP, Stooffel PJ. The preparation and bacteriostatic activity of substituted ureas. J Am Chem Soc. 1957 Mar; 79 (5): 1236-1245. [ Links ]
18. Black JG, Howes D, Rutherford T. Skin deposition and penetration of trichlorocarbanilide. Toxicology. 1975 Feb; 3 (2): 253-264. [ Links ]
19. Sosnik A, Carcaboso AM, Chiappetta DA. Polymeric Nanocarriers: New endeavors for the optimization of the technological aspects of drugs. Recent Pat Biomed Eng. 2008 Jan; 1 (1): 43-59. [ Links ]
20. Segewicz L, Petrowsky M. editors. Polymer Aging, Stabilizers and Amphiphilic Block Copolymers. New York, USA: Nova Publishers; 2010. Chapter 5, Chiappetta DA, Degrossi J, Lizarazo RA, Salinas DL, Martínez F, Sosnik A. Molecular implications in the solubilization of the antibacterial agent Triclocarban by means of branched poly(ethylene oxide)-poly(propylene oxide) polymeric micelles; 373 p. [ Links ]
21. McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS. Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem. 2002 Jul; 21 (7): 1323-1329. [ Links ]
22. Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V. Aquatic toxicity of triclosan. Environ Toxicol Chem. 2002 Jul; 21 (7): 1338-1349. [ Links ]
23. Aragón DM, Ruidiaz MA, Vargas EF, Bregni C, Chiappetta DA, Sosnik A, et al. Solubility at several temperatures of the antimicrobial agent triclosan in organic solvents of different hydrogen bonding capability. J Chem Eng Data. 2008 Nov; 53 (11): 2576-2580. [ Links ]
24. Delgado DR, Sosnik A, Martínez F. Transfer thermodynamics of triclosan from water to organic solvents with different hydrogen bonding capability. Latin Am J Pharm. 2011; 30 (3): 459-466. [ Links ]
25. Aragón DM, Sosnik A, Martínez F. Solution thermodynamics of triclocarban in some organic solvents of different hydrogen bonding capability. J Solution Chem. 2009 Dec; 38 (12): 1493- 1503. [ Links ]
26. Tewes F, Boury F, Benoit JP. Biodegradable microspheres: Advances in production technology. In: Benita S, Editor. Microencapsulation: Methods and Industrial Applications. 2nd ed. New York, USA: Taylor & Francis Group, LLC; 2006. p. 1-53. [ Links ]
27. US Pharmacopeia 26th ed. United States Pharmacopeial Convention. Rockville, MD, USA; 2002. [ Links ]
28. Budavari S, O'Neil MJ, Smith A, Heckelman PE, Obenchain Jr. JR, Gallipeau JAR, et al. The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th ed. Whitehouse Station, NJ, USA: Merck & Co., Inc.; 2001. 1721 p. [ Links ]
29. Veiga MD, Merino M, Cirri M, Maestrelli F, Mura P. Comparative study on triclosan interactions in solution and in the solid state with natural and chemically modified cyclodextrins. J Incl Phenom Macro. 2005 Feb; 53 (1-2): 77-83. [ Links ]
30. Hildebrand JH, Prausnitz JM, Scott RL. Regular and Related Solutions. New York, USA: Van Nostrand Reinhold; 1970. 240 p. [ Links ]
31. Hansen CM. The three dimensional solubility parameter-key to paint component affinities I, Solvents, plasticizers, polymers and resins. J Paint Technol. 1967; 39 (505): 104-117. [ Links ]
32. Barton A. Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed. New York, USA: CRC Press; 1991. p. 69-156. [ Links ]
33. Aragón DM, Chiappetta DA, Degrossi J, Vargas EF, Bregni C, Sosnik A, et al. Gibbs energy of transfer processes for the antimicrobial agent Triclosan from water to some organic solvents at 25.0 °C. Rev Colomb Cienc Quím Farm. 2008 Dec; 37 (2): 241-257. [ Links ]
34. Martin A, Bustamante P. El parámetro de solubilidad en las ciencias farmacéuticas. Anal Real Acad Farm. 1989 Jun; 55 (2): 175-202. [ Links ]
35. Martin A, Bustamante P, Chun AHC. Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences. 4th ed. Philadelphia, USA: Lea & Febiger; 1993. p. 225. [ Links ]
36. Mora CP, Martínez F. Thermodynamic quantities relative to solution processes of naproxen in aqueous media at pH 1.2 and 7.4. Phys Chem Liq. 2006 Oct; 44 (5): 585-596. [ Links ]
37. Kristl A, Vesnaver G. Thermodynamic investigation of the effect of octanol-water mutual miscibility on the partitioning and solubility of some guanine derivatives. J Chem Soc, Faraday Trans. 1995 Jun; 91 (6): 995-998. [ Links ]
38. Krug RR, Hunter WG, Grieger RA. Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effects. J Phys Chem. 1976 Oct; 80 (21): 2341-2351. [ Links ]
39. Bustamante P, Romero S, Peña A, Escalera B, Reillo A. Nonlinear enthalpy-entropy compensation for the solubility of drugs in solvent mixtures: paracetamol, acetanilide and nalidixic acid in dioxane-water. J Pharm Sci. 1998 Dec; 87 (12): 1590-1596. [ Links ]
40. Bevington PR. Data Reduction and Error Analysis for the Physical Sciences. New York, USA: McGraw-Hill Book Co.; 1969. p. 56-91. [ Links ]
41. Perlovich GL, Kurkov SV, Kinchin AN, Bauer-Brandl A. Thermodynamics of solutions III: Comparison of the solvation of (+)-naproxen with other NSAIDs. Eur J Pharm Biopharm. 2004 Mar; 57 (2): 411-420. [ Links ]
42. Aragón DM, Rosas JE, Martínez F. Solution thermodynamics of naproxen in some volatile organic solvents. Phys Chem Liq. 2010 Aug; 48 (4): 437-449. [ Links ]
43. Aragón DM, Rosas JE, Martínez F. Thermodynamic study of the solubility of ibuprofen in acetone and dichloromethane. Braz J Pharm Sci. 2010 Jun; 46 (2): 227-235. [ Links ]
44. Tanford C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes. New York, USA: John Wiley & Sons; 1973. 234 p. [ Links ]
45. Sangster J. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry. Chichester, UK: John Wiley & Sons; 1997. p. 1-55. [ Links ]