Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.19 no.2 Medellín May/Aug. 2012
FOODS: SCIENCE, TECHNOLOGY AND ENGINEERING
MODIFIED ARRACACHA STARCH FILMS CHARACTERIZATION AND ITS POTENTIAL UTILIZATION AS FOOD PACKAGING
CARACTERIZACIÓN DE PELÍCULAS DE ALMIDÓN MODIFICADO DE ARRACACHA Y SU EVENTUAL APLICACIÓN COMO EMPAQUE DE ALIMENTOS
Oscar J. MEDINA V. Ph.D.1; Oscar H. PARDO C. M. Sc.1*; Cesar A. ORTIZ M. Sc.2
1 Grupo de investigación Química y Tecnología de Alimentos (GQTA). Universidad Pedagógica y Tecnológica de Colombia. Avenida Norte, Km 2, Tunja-Boyacá, Colombia.
2 Grupo de investigación en Grupo de Investigación en Superficies Electroquímica y Corrosión (GSEC). Universidad Pedagógica y Tecnológica de Colombia. Avenida Norte, Km 2, Tunja-Boyacá, Colombia.
* Autor a quien se debe dirigir la correspondencia: oscarhernando.pardo@uptc.edu.co.
Received: 04 November 2011 Accepted: 28 August 2012
ABSTRACT
Background: The use of petroleum-based plastics has increased in recent years. These materials are resistant and economically competitive. However, the environmental pollution caused by these is very high. For this reason, some research has focused on the alleviation of this environmental problem, mainly through the development and use of biodegradable polymers. The food industry as an economic dynamic sector is looking for new environmental and secure alternatives for the consumer welfare. In packaging sector, materials should be renewable and final products must be recyclable, innovative and economically competitive. New starch sources could be an adequate alternative. Objetive: The main objective in this research project was to evaluate the chemical modification of arracacha starch, testing different plasticizer concentrations, as a raw material for food biodegradable packaging production. Methods: The arracacha native starch was acetylated or oxidized to produce biodegradable films. The starches were characterized by infrared spectroscopy, scanning electron microscopy, x-ray diffraction and volumetric techniques. Results: Films made with acetylated starch presented more transparency. Native starch films had lower water solubility and greater stability in acid and alkaline conditions. The effect of alkaline conditions was higher than the acid conditions for the three types of films in all treatments. Conclusion: Physicochemical properties of the films were directly influenced by the amount of plasticizer used and by the starch modification type. The physicochemical and microbiological tests of the meat show the possible use of starch films for packaging.
Keywords: Starch, acetylated, oxidized, plasticizer, films.
RESUMEN
Antecedentes: El uso de plásticos derivados del petróleo se ha incrementado en los últimos años. Estos materiales son resistentes y económicamente competitivos. Sin embargo, la contaminación ambiental causada por estos es muy alta. Por esta razón, algunas investigaciones se han centrado en la mitigación de este problema ambiental, principalmente a través del desarrollo y la utilización de polímeros biodegradables. La industria de la alimentación como un sector económico dinámico, está buscando alternativas ambientales y seguras para el bienestar de los consumidores. En el sector de envases, los materiales deben ser renovables, reciclables, innovadores y competitivos; las nuevas fuentes de almidón pueden ser una alternativa. Objetivo: El objetivo de esta investigación fue evaluar la modificación química del almidón de arracacha y variar la concentración del plastificante como materia prima para la producción de películas biodegradables para empaques de alimentos. Métodos: El almidón nativo de arracacha se acetiló u oxidó para producir películas biodegradables. Los almidones fueron caracterizados por espectroscopía infrarroja, microscopía electrónica de barrido, difracción de rayos-x y técnicas volumétricas. Resultados: Las películas obtenidas con almidón acetilado presentaron más transparencia. Las películas de almidón nativo tuvieron una menor solubilidad en agua y una mayor estabilidad en condiciones ácidas y alcalinas. El efecto de las condiciones alcalinas fue mayor que las condiciones ácidas para los tres tipos de películas en todos los tratamientos. Conclusión: Las propiedades fisicoquímicas de las películas fueron directamente influenciados por la cantidad de plastificante utilizado y por el tipo de modificación del almidón. Las pruebas fisicoquímicas y microbiológicas de la carne muestran la posible utilización de películas de almidón como material de empaque.
Palabras clave: Almidón, acetilado, oxidados, plastificantes, películas.
INTRODUCTION
According to recent reports, more than 150 million tons of plastic are produced worldwide every year (1). The pollution caused by these materials is very high, so research activities have focused to alleviate this environmental problem, mainly through the development and use of biodegradable polymers (2).
Biodegradable polymers are used and tested in a large number of applications such as packaging, paper production, fibers, and biomedical applications as implants and controlled release of medicines (3). These polymers must be biodegradable and nontoxic and must have good chemical, mechanical, thermal and rheological properties. In packaging sector, materials should be renewable and final products must be recyclable, innovative and economically competitive (4).
However, only a few monomers and polymers accomplish these requirements. Among most common polymers, starch meets most of these rigorous requirements. Starch essentially consists of a mixture of polysaccharides mainly amylose, amylopectin, and a minor fraction (1% -2%) with non glucosidic conformation (5). Most of the starches in the glucosidic structure are composed of 20% amylose and the remaining 80% amylopectin (6).
Traditionally, Starch is obtained from grains of cereals, seeds of legumes, tubers, and corn is the main source (7-9). However, functional, rheological, and physicochemical properties of starches from non-conventional sources have been studied as well (10).
Several studies have focused on the use of starch in obtaining biodegradable films as a product with non-toxic characteristics, low-cost, abundant raw material, and relatively easy handling (11-13). However, the use of native starches is limited to the condition of processing (temperature, pH and pressure) which reduces their use in industrial applications (14). In addition, the use of the unmodified starch is also limited due to its fragility, when used as packaging material, but also because of the deterioration of mechanical properties at environmental conditions by exposure to moisture, its reduced processability due to its high viscosity and its incompatibility with some solvents and polymers (15, 16).
In order to improve its characteristics, several modifications are carried out with starch, by using three methods: physicochemical reactions, microbial type changes, or a combination of these (6).
Plastics with high content of native starch are highly hydrophilic and quickly disintegrable in contact with water (13). Modif ied starches with lower levels of chemical modification can signif icantly improve their hydrophobicity. Likewise, they change their chemical, physical and rheological properties. The introduction of a polysaccharide ester group is an important development that allows changes in the hydrophilic nature and produces significant changes in the mechanical and thermal properties (6).
This work focuses on the chemical modification of arracacha starch by using two methods (acetylation and oxidation), its suitability as materials for making biodegradable films, with the best physicochemical properties, and its application as food packaging material. It is worth to bear in mind that this root has considerably higher starch content (17), and that Colombia is the largest arracacha producer in the world (18).
MATERIALS AND METHODS
Mature arracacha (Arracacia xanthorrhiza Bancroft) samples, variety yellow, were obtained from La Paz (Santander, Colombia). Arracacha starch was extracted according to the methodology proposed by Aristizábal and Sánchez (19), with some modifications. All reagents used were analytical grade (Merck®, Carlo Erba® and J.T. Baker®).
Arracacha starch acetylation
Acetylated Starch (AS) was obtained with the methodology described by Phillips et al., 1999 (20), with some modifications. 50 g of native starch (NS) in dry dock were suspended in 250 mL of distilled water, pH was adjusted to 8.4 and the solutions were shaken constantly during 30 min. 4.2 g of acetic anhydride were slowly added and pH was maintained between 8.0-8.4. After adjusting pH to 4.5 with HCl 0.5 eq-g L-1, samples were centrifuged and the precipitates were washed four times with distilled water, dried at 35 ± 2°C on stove, during 48 h, and finally, the obtained starch was milled in a IKA® A11 basic S1 miller.
Arracacha starch oxidation
Oxidized starch (OS) was obtained by following the methodology proposed by Wang et al., 2003 (21), with some modifications. A 20% (w/v) starch suspension was prepared shaking constantly at 35°C during 15 min. Then, pH was adjusted to 9.5 with NaOH 0.3 eq-g L-1and NaOCl 2.0% of active chlorine (p/v) was added, maintaining pH at 9.5 with NaOH 0.3 eq-g L-1. The reaction was left for one hour more. Afterwards, pH was neutralized to 7.0 with HCl 0.3 eq-g L-1, twice washed with distilled water and dried in oven at 35 ± 2°C during 48 h, and finally, the obtained starch was milled in a IKA® A11 basic S1 miller.
Determination of the substitution degree
For the determination of the AS substitution degree, the percentage of acetyl groups was firstly estimated. This percentage was based on the titration of acetyl residues after the sample alkaline treatment (22). 1 g of AS (dry base) in a 250 mL Erlenmeyer flask was weighted. 50 mL of 75% ethanol were added and then placed in a water bath at 50°C, shaking during 30 min. The sample was cooled to room temperature, 40 mL of KOH 0.5 eq-g L-1 and a few drops of phenolphthalein were added, shaking it throughout the process. The flask was covered and shaken for 72 h more. After this, the saponified sample was titled with HCl 0.5 eq-g L-1, and kept at rest during 2 h. The additional alkali which could be leachate from sample was titled. Same procedure was carried out with the NS in order to use it as a reference. The percentage of groups CH3-C = O was calculated as follows:
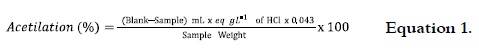
where: Blank = mL of HCl used in the NS titration; Sample = mL of HCl used in the AS titration; 0.043 = milliequivalents of the acetyl group.
In AS the substitution degree (DS) is equivalent to the average number of hydroxyl groups replaced by groups CH3-C = O in the anhydrous glucose unit (AGU), and was calculated as follows:
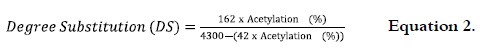
where: 162 = molecular weight of glucose, 4300 = 100 x molecular weight of the acetyl group, 42 = molecular weight of the acetyl group -1.
Determination of Carbonyl groups
The percentage of Carbonyl groups in OS was calculated according to the methodology of Smith (23). 4 g of NS were suspended in 100 mL of distilled water. The suspension was boiled for 20 minutes, cooled to 40°C and pH was adjusted to 3.2 with HCl 0.1 eq-g L-1. 15 mL of hydroxylamine were added and kept in slow shaking for 4 hours. The excess of hydroxylamine was titled quickly with HCl 0.1 eq-g L-1 till pH 3.2. A blank with hydroxylamine reagent was used. Carbonyl content was calculated as follows:
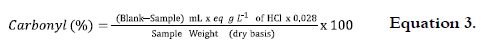
where: Blank = mL of HCl used in the NS titration; Sample = mL of HCl used in the AS titration; 0.028 = milliequivalents of the carbonyl group.
Determination of carboxyl groups
The percentage of the carboxyl groups in OS was determined by following the methodology of Chattopadhyay et al., 1997 (24). 2 g of NS were weighted and 25 mL of HCl 0.1 eq-g L-1 were added. The sample was kept under stirring for 30 min. Sample was vacuum filtered using filters Watman No. 4 and washed with 400 mL of distilled water. Starch paste was transferred to a 500 mL beaker, and 300 mL of distilled water was added. It was warmed in a bath with boiling water along with constant agitation during 20 min. 150 mL of distilled water were added to the dispersion of hot starch adjusting pH to 8.3 with standardized NaOH 0.01 eq-g L-1. A blank with NS was used and carboxyl content was calculated as follows:

where: Blank = mL of NaOH used in the NS titration, Sample = mL of NaOH used in the AS titration, 0.0453 = milliequivalents of the carboxyl group.
Infrared spectroscopy (FTIR)
NS, OS and AS Infrared spectra were obtained with a Prestige-21 SHIMADZU spectrophotometer. The FTIR analysis was performed using a Prestige-21 SHIMADZU spectrometer. The NS, OS and AS samples were collected using the KBr pellet method. FTIR spectra were recorded at a resolution of 4 cm-1 and 32 scans. Wave number ranged between 750 and 3500 cm-1. The NS, AS and OS were equilibrated at 50°C to a constant moisture (10%) prior to analysis.
Diffraction of X-rays (DRX)
The X-ray diffraction was analyzed using a XPERT-PRO PANalytical diffractometer, a conventional copper target X-ray tube set to 40 kV and 40 mA. The X-ray source was Cu Kα-1 radiation and data were collected from 2θ of 5° to 45° (θ being the angle of diffraction) with a step width of 0.02°, at room temperature. The NS, AS and OS were equilibrated at 50°C to a constant moisture (10%) prior to analysis.
Scanning electron microscopy (SEM)
The morphology of the NS, OS and AS samples was observed in a LEO-430 microscope. Samples were placed in a double adhesive tape and coated with carbon at vacuum conditions and 2000x micrographs were captured.
Preparation of starch films
AS, OS and AS films were prepared by suspending 2 g of starch in a mixture of distilled water and glycerol in different proportions, shaken at 500 r.p.m at room temperature for 20 min. The suspension was treated with gelatinization process for 15 min. The mixture was poured into moulds of 30 x 15 cm steel and left to dry at room temperature for 5 days. Followed by a full factorial design AxB, where A is the amount of glycerol with 5 levels (A: 0.6; B: 0.8; C: 1.0; D: 1.2; E: 1.4 mL), and B the type of starches with 3 levels (AS, OS y NS). Combinations (treatments) were considered in total 15.
Physicochemical properties
For the characterization of films, the methodology proposed by Hu et al., 2009 (1), was followed with some modifications.
Transparency films
The transparency value of the films of NS, OS and AS was obtained by measuring its transmittance in a spectrophotometer Thermo GENESYS 10uV at a wavelength of 800 nm. Samples were tested in triplicate.
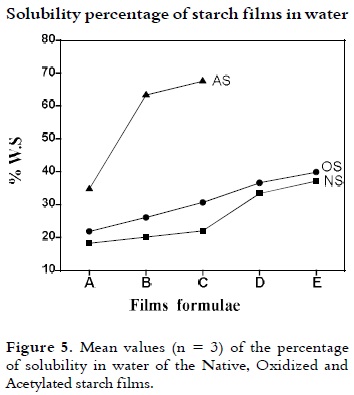
Solubility percentage of starch films in water
Samples with a size of 4 x 2 cm for each NS, OS and AS films were dried up to 40°C until reach constant weight (Wo), submerged in a beaker with 50 mL of distilled water, and the covered vessels staged at 27°C with some agitation for 24 h. After this time, samples were released and dried up to 40°C until reach constant weight (W2). Percentage of water solubility (% WS) was calculated as follows:
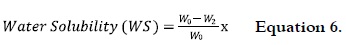
Samples were tested in triplicate.
Stability in Alkaline conditions
Samples with a size of 4x4 cm for each NS, OS and AS films were immersed in sealed glass container with 30 mL of NaOH 0.1 eq-g L-1 maintaining the temperature at 25°C with eventual stirring. The stability of the films was determined by observing changes in appearance for 60 days and a photographic record was kept.
Stability in acid conditions
Samples with a size of 4x4 cm for each NS, OS and AS f ilms were immersed in sealed glass container with 30 mL of HCl 0.1 eq-g L-1 maintaining the temperature at 25°C with eventual stirring. The stability of the films was determined by observing changes in appearance for 60 days and a photographic record was kept.
Arracacha starch films used as packaging of meat
Taking into account all characteristics showed by the NS films of formulation C, these were selected for an experiment of application as packing material, as follows:
Two cuts of the same size with a weight of 50 gr of lean beef tenderloin from a healthy calf Normand race 2 years old were packed separately. Another cut of meat with the same characteristics was packed in a commercial plastic bag (Vinipel®). All the samples were stored at refrigeration temperature between 4°C ± 1. To evaluate the effectiveness of arracacha starch films as packing material from the physicochemical and microbiological properties analysis of meat, all the cuts were monitored every three days during a period of 12 days.
pH and titratable acidity determination
The pH was determined by potentiometric method AOAC 945.10 (25) and titratable acidity by volumetric method AOAC 942.15. (26).
Fecal coliforms most probable number (MPN)
The MPN was determined according to the Colombian Technical Norm (NTC 4516). (27)
Statistical analysis
Analysis of variance with the statistical program SPSS version 17 was performed; when significant differences were found, the Tukey test was used (p < 0. 05).
RESULTS
Physicochemical characterization of films
The following are the results of physicochemical trials of five samples from each one of the classes of starch, except for the AS films for which D and E formulations did not allow conducting any tests.
Arracacha starch films used as packaging of meat
pH and titratable acidity determination
Fecal coliforms most probable number (MPN)
Meat extracts packaged in commercial plastic as well as in bags made from NS, formulation C, were diluted at 10-1, 10-2 and 10-3. Of each one, 1 mL was extracted and then inoculated in 3 tubes containing Brilla Broth. All these 3 series of 3 tubes each one, were incubated at 37°C during 48 h. After this, nor gas presence neither turbidity were evidenced in Durham bell, so they were marbled as negatives (-).
DISCUSSION
Characteristics of the starches used in the films preparation
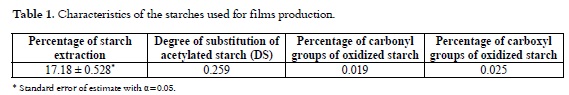
The Percentage of starch extraction matches the value reported by Hermann et al., 1997 (28). Despite of the diversity of its varieties, arracacha starch extraction has been reported as well as its quantification methods. After the esterification reaction was performed, AS was obtained with a percentage of acetyl groups of 6.45%, which equates to a 0.259 DS, (Table 1). This means that from the whole OH functional groups, 0.259 were replaced by acetyls. Similar results have been reported by Singh et al., 2004 (29). The percentage of carbonyl and carboxyl groups of oxidized starch OS was obtained with a value of 0.019 carbonyl groups and a percentage of 0.025 carboxyl groups. Similar results were reported by Sandhua et al., 2008 (30) and Rivas et al., 2008 (31), (Table 1).
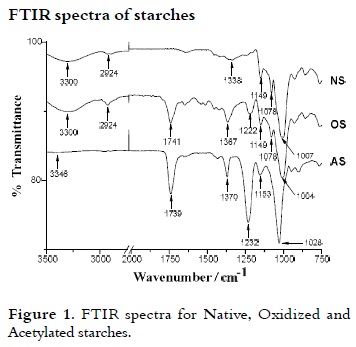
Infrared spectroscopy (FTIR) of starches
Figure 1 shows the spectrum FTIR of NS, whose bands are characteristic for this kind of compounds (32 - 34). The vibration of the hydroxyl group appears as a broad band at 3300 cm-1. The vibration of the C-O bond of secondary hydroxyls appears at 1007 cm-1 and the C-O-C vibration appears at 1149 cm-1; tension bands corresponding to OH, are located at 1338 cm-1, and the tension band ''out of plane'' corresponding to CH is found at 1078 cm-1.
The FTIR spectrum of the OS in which a new band appears at 1741 cm-1 corresponding to the vibration of the carbonyl bond formed by the oxidation reaction is showed. Two new bands at 1367 cm-1 and 1222 cm-1 appear, corresponding to the tension of the C-O-H and C-O bonds respectively, formed during oxidation of starch from the carboxylic groups.
Two new bands appear in the AS FTIR spectrum: 1739 cm-1, that is attributed to the vibration of the carbonyl group, formed by the reaction of acetylation of hydroxyl groups and other at 1232 cm-1, that is characteristic of tension vibration of the ester function C-O bonds. The vibration of the tension of O-C-C bonds of esters appears at 1153 cm-1. The vibration of bending of the methyl group of the formed ester appears at 1370 cm-1. Finally, the intensity of the band corresponding to the OH group decreases considerably due to the high degree of substitution.
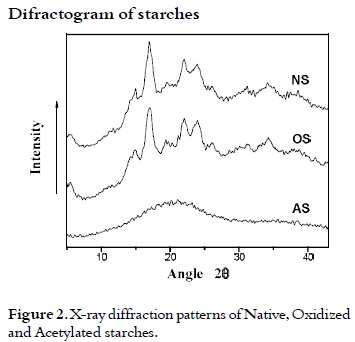
X-rays diffraction (DRX) of starches
Figure 2 shows the diffraction patterns of the three types of starches. The diffraction pattern of native starch of arracacha showed a β type crystal structure with peaks at 5, 15, 17, 20, 22 and 24 at the angle (2θ), indicating a typical pattern of arracacha starch similar to the potato starch (35). The oxidation reaction did not affect the X-ray diffraction pattern, but the percentage of crystallinity of starch (31). It is 12.88% for the OS whereas for the NS is 11.08%. The increase of crystallinity for OS could be due to depolymerization reactions presumably, as a secondary effect of the oxidation reaction (36), obtaining amylose chains which undergo a certain order degree (37).
During the acetylation reaction, the AS semicrystalline structure affected largely the x-ray diffraction pattern, and a new structure of amorphous nature is observed. For AS, crystallinity loss may have been due to the acetylation reaction on the amylopectin, reducing its order degree, due to the obtained high degree of substitution. Similar results have been reported by Hui Chi et al., 2008 (38).
Scanning electron microscopy (SEM) of starches
The micrograph of NS reveals particles whose sizes vary from 5 to 9 µm the smallest ones, and from 12.4 to 15 µm the largest ones. The form of granules tends to be rounded, but it also observed morphological irregularities (cuboids or deformed pyramids) and other truncated forms, probably originated during the starch extraction process. During the Oxidation reaction, morphology suffers no obvious changes except the greater tendency to the clustering. In the OS morphology there are fewer tendencies to form conglomerates of particles forming clusters (See Figure 3).
The acetylation reaction causes a disruption of the matrix, which is evidenced by a greater agglomeration of the granules and a modification of their size, shape and texture. In these conditions, the AS probably suffers destructuration as it interacts with amylose and amylopectin, resulting in a different sort of chemical bonds. The size of the agglomerates in the AS reveals a different type of intergranular cohesion, which may be caused by changes in functional groups, which, in turn, results in increasing hydrogen bonds and a subsequent intergranular agglomeration (29).
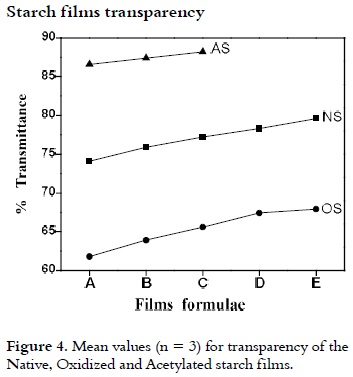
Films Transparency
In Figure 4, is shown the average of the transmittance values as indicator of f ilms transparency for each one of the three types of films with different glycerol concentrations. It was observed that at higher glycerol content, greater transparency of NS, OS and AS films was observed. The greatest transparency value was obtained for the AS films. Thus, starch increases its structural water retention capacity, which facilitates the crossing of light. Furthermore, the lowest transparency of OS films may be due to the oxidation reaction, when starch undergoes a whitening process, which is maintained during the gelling step. This makes OS films more opaque.
Solubility percentage of starch films in water
The percentage of the films water solubility (% WS) is shown in Figure 5. It shows that when the films glycerol amount is increased, its solubility increases as well, due to the hydrophilic nature of this compound (39). On average, the films made with NS showed a lower solubility in water than films with OS and AS. The NS and OS films water solubility is very similar. During the oxidation reaction, starch undergoes partial hydrolysis which shortens the length of the chains of glucose and increases its solubility.
Probably, the increasing in the AS films water solubility is due to the reaction of acetylation which induces greater water absorption of starch granules. In this case, the substituent group acts as a spacer, preventing that starch chains become closer. During the solubility test at 25°C and under agitation, the trapped water gets out and this phenomenon causes a greater loss of weight.
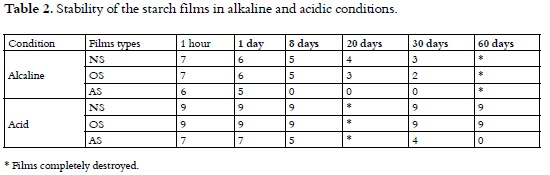
Stability in alkaline and acid conditions of starch films
Taking into account that films could be used as packaging and therefore they would eventually be exposed to acidic or alkaline conditions, a test and a photographic record of the stability of the films in acid and alkaline conditions were conducted.
Stability, both in alkaline and acid conditions, was measured on a scale from 0 (stable) to 10 (unstable), at 1 hour, 1 day, 8 days, 20 days, 30 days and additionally in acid conditions at 60 days. The average results of the five films test score with each starch type are shown in Table 2 and some samples of the photographic record are included in Figure 6.
In alkaline condition and one hour later of the test beginning, NS, OS and AS films showed swelling and size increasing. One day later, the three types of films maintained their swelling and began to show breakdown also; these characteristics were more evident in the AS films.
Eight days later, NS films breakdown increased while OS films breakdown continued and AS films were completely destroyed (Figure 6A). Twenty days later NS films were stable yet, while OS films showed total breakdown (Figure 6B). Thirty days later NS films began to breakdown (Figure 6C).
Probably, the low stability of the three types of f ilms in alkaline conditions may be due to that sodium hydroxide reacted with the hydroxyl groups of the films starch molecules and broke the hydrogen bonds, which would cause weakness of the intra and inter molecular interactions. However, the smaller stability of the OS films may be due to that sodium ions react with carboxyl groups to form carboxilates, which would increase the hydrophilic nature and therefore the starch solubility (1).
On the other hand, in AS ester bonds could undergo some degree of hydrolysis caused by sodium hydroxide. This reaction facilitates that the starch hydroxyl groups be formed again, besides of the formation of sodium acetate; this one has high solubility in water. Consequently, the AS films solubility is greater than NS and OS ones; this fact explains its greater instability.
In acid conditions and one hour after the test beginning, the NS and OS films size, increased slightly, while the AS films showed high swelling; one day after, the three types of films maintained these same characteristics.
Eight Days later, the AS film began to show breakdown (Figure 7A). Although Thirty days after, NS and OS films remained stable, the AS films showed greater breakdown (Figure 7B) and 60 days later, NS and OS films maintained stable and AS films showed total breakdown (Figure 7C). Acid conditions do not affect considerably NS and OS films stability.
AS films stability is lower possibly due to the ester group acid hydrolysis reactions which retrieve original starch hydroxyl groups. On the other hand, this hydrolysis reaction produces acetic acid also, which has higher solubility in water and therefore increases the instability of the films made with this type of starch.
Arracacha starch films used as packaging of meat
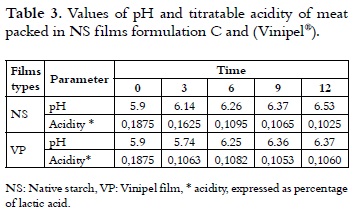
pH and titratable acidity determination
The initial pH and acidity of the meat are within the characteristic values for these products (40). As time goes by, the pH of the meat increases while acidity values decrease, due to increasing of basic character of compounds derived from the hydrolysis of the meat proteins, as can be seen in Table 3. There is no major difference in these physicochemical parameters, when commercial plastic (Vinipel®) or NS films of formulation C, are used as packing material.
Fecal coliforms most probable number (MPN)
These results confirm absence of fecal coliforms and a hygienic product as well. The results obtained indicate that NS films, formulation C, do not permit any contamination with microorganisms to the meat sample, compared to the reference material used.
CONCLUSIONS
AS films showed greater transparency and water solubility than NS and OS films, at the same glycerol amounts. NS Films showed greater stability in acid and alkaline conditions than AS and OS films. In all treatments, for the three types of films, the effect of the alkaline condition was greater than the acid one. The films physicochemical properties are directly influenced by the plasticizer concentration and by the type of starch used for their production. From the laboratory analysis report, is possible to asseverate that films made from arracacha starch are suitable for being used as meat packaging. However, these films experiment a degradation process due to the crystallization of water after 10 days of use.
REFERENCES
1. Hu G, Chen J, Gao J. Preparation and characteristics of oxidized potato starch films. [ Links ]
2. Carbohydr Polym. 2009 Mar; 76 (2): 291-298. [ Links ]
3. Merchan JP, Ballesteros D, Jiménez IC, Jiménez JA, Medina AO. Estudio de la Biodegradación Aerobia de Almidón Termopl ástico (TPS). Suplemento de la RLMM. 2009 Jul; 1 (1): 39-44. [ Links ]
4. Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999 Jun; 99 (11): 3181-3198. [ Links ]
5. Tuominen J. Chain linked acid polymers: polymerization degradation studies [dissertation]. [Helsinki]: Technology University of Helsinki, 2003. 54 p. [ Links ]
6. BeMiller JN, Whistler RL. Starch Chemistry and Technology. 2nd ed. London, UK: Academic Press Publications; 1984. 879 p. [ Links ]
7. Peñaranda OI, Perilla JE, Algecira NA. A review of using organic acids to chemically modify starch. Ingeniería e Investigaci ón. 2008 Dec; 28 (3): 47-52. [ Links ]
8. Waliszewski KW, Aparicio MA, Bello LA, Monroy JA. Changes of banana starch by chemical and physical modification. Carbohydr Polym. 2003 May; 52 (3): 237-242. [ Links ]
9. Alarcón M, Dufour D. Almidón agrio de yuca en Colombia, Tomo I Producción y Recomendaciones. 1era ed. Cali, Colombia: CIAT; 1998. 62 p. [ Links ]
10. Medina JA, Salas JC. Morphological Characterization of Native starch Granule: Apperance, Shape, Size and its Distribution. Rev Ing. 2003 Jun; 27: 56-62. [ Links ]
11. Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym. 2001 Apr; 45 (3): 253-267. [ Links ]
12. Lourdin DV, Colonna P. Influence of amylase content on starch films and foams. Carbohydr Polym. 1995 Jan; 27 (4): 261-270. [ Links ]
13. García MA, Martino MN, Zaritzky NE. Lipid addition to improve barrier properties of edible starch-based films and coatings. J Food Science. 2000 Sep; 65 (6): 941-947. [ Links ]
14. García MA, Pinotti M, Zaritzky NM. Characterization of composite hydrocolloid films. Carbohydr Polym. 2004 Jul; 56 (3): 339-345. [ Links ]
15. Zamudio FP, Bello PL, Vargas TA, Hernández UJ, Romero BC. Partial characterization of films prepared with oxidized banana starch. Agrociencia. 2007 Sep; 41 (8): 837-844. [ Links ]
16. Fang JM, Fowler PA, Tomkinson J, Hill CA. An investigation of the use of recovered vegetable oil for the preparation of starch thermoplastics. Carbohydr Polym. 2002 Dec; 50 (4): 429-434. [ Links ]
17. Gong Q, Wang LQ, Tu K. In situ polymerization of starch with lactic acid in aqueous solution and the microstructure characterization. Carbohydr Polym. 2006 Jun; 64 (4): 501-509. [ Links ]
18. Jiménez FS. Características nutricionales de la arracacha (Arracacia xanthorrhiza) y sus perspectivas en la alimentación [Internet]. Lima (PE): Red peruana de alimentación y nutrición; 2005 Jan (cited 2010 Dec 15). Available from: http://www.slideshare.net/kevin1990/caracteristicas-nutricionales-de-la-arrecacha. [ Links ]
19. Rodríguez G. Concepción de un modelo de agroindustria rural para la elaboración de harina y almidón a partir de raíces y tubérculos promisorios, con énfasis en los casos de achira (Canna edulis), arracacha (Arracacia xanthorriza) y ñame (Dioscorea sp.) [Internet]. Tibaitatá (CO): CORPOICA.PRONATTA; 2003 Dec (cited 2010 Oct 18). Available from: http://www.infoandina.org/system/files/recursos/AIRachira.pdf. [ Links ]
20. Aristizábal J, Sánchez T. Guía técnica para producción y análisis de almidón de yuca. 1era ed. Roma, Italia: FAO; 2007. 104 p. [ Links ]
21. Phillips DL, Huijum L, Duohai P, Harold C. General application of Raman spectroscopy for the determination of level of acetylation in modified starches. Cereal Chem. 1999 Feb; 76 (3): 439-443. [ Links ]
22. Wang YJ, Wang L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr Polym. 2003 May; 52 (3): 207-217. [ Links ]
23. Wurzburg OB. Methods in Carbohydrate Chemistry. 1st ed. New York, USA: Academic Press; 1964. 240-241 p. [ Links ]
24. Smith RJ. Starch chemistry and technology. 3rd ed. New York, USA: Academic Press; 2009. 620-625 p. [ Links ]
25. Chattopadhyay S, Singhal RS, Kulkarni PR. Optimization of conditions of synthesis of oxidized starch from corn and amaranth for use in film-forming applications. Carbohydr Polym. 1997 Dec; 34 (4): 203-212. [ Links ]
26. Official Methods of Analysis. Association of Official Analytical Chemists (AOAC). Potentiometric method, Number 945.10. 18th ed. Washington D.C, USA: AOAC; 2000. [ Links ]
27. Official Methods of Analysis. Association of Official Analytical Chemists (AOAC). Potentiometric method, Number 942.15. 18th ed. Washington D.C, USA: AOAC; 2000. [ Links ]
28. Instituto Colombiano de Normas Técnicas y Certificación. Norma Técnica Colombiana. NTC 4516. Primera actualización. Bogotá: ICONTEC; 2009. [ Links ]
29. Hermann H, Uptmoor R, Freire I, Montalvo J. International Potato Centre Program Report 1995-1996: Crop Growth and Starch Productivity of Edible Canna [Internet]. Lima, (PE): Partnership by Josephine Dell' Orco Prain; 1997 Sep (cited 2010 Nov 21). Available from: http://books.google.com.co/books?id=6mE35sBhT48Cdq=Crop+Growth+and+Starch+Productivity+of+Edible+Cannahl=essource=gbs_navlinks_s. [ Links ]
30. Singh N, Chawla D, Singh J. Inf luence of acetic anhydride on physicochemical, morphological and thermal properties of corn and potato starch. Food Chem. 2004 Aug; 86 (4): 601-608. [ Links ]
31. Sandhua KS, Kaura M, Singhb N, Lim ST. A comparison of native and oxidized normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. LWT. 2008 Jul; 41(6): 1000-1010. [ Links ]
32. Rivas GM, Montealvo MM, Sánchez RM, Núñez SM, Bello PL. Morphological Molecular and Physicochemical Characterization of Oxidized and Lintnerized Banana Starch. Agrociencia. 2008 Jul-Aug; 42 (5): 487-497. [ Links ]
33. Dumoulin Y, Alex S, Szabo P, Cartilier L, Alexandru M. Cross linked amylose as matrix for drug controlled release. X-ray and FT-IR structural analysis. Carbohydr Polym. 1998 Dec; 37 (4): 361-370. [ Links ]
34. Dragunski DC, Pawlicka A. Preparation and characterization of Starch Grafted with Toluene Poly (propylene oxide) diisocyanate. Materials Research. 2001 Apr; 4 (2): 77-81. [ Links ]
35. Kacurakova M, Wilson R.H. Developments in mid-infrared FT-IR spectroscopy of seleted carbohydrates. Carbohydr Polym. 2001 Apr; 44 (4): 291-303. [ Links ]
36. Mathew S, Adlercreutz P. Mediator facilitated, laccase catalysed oxidation of granular potato starch and the physico-chemical characterisation of the oxidized products. Bioresour Technol. 2009 Jul; 100 (14): 3576-3584. [ Links ]
37. Kuakpetoon D, Wang Y. Locations of hypochlorite oxidation in corn starches varying in amylose content. Carbohydr Res. 2008 Jan; 343 (1): 90-100. [ Links ]
38. Morrison WR, Tester RF, Gidley MJ, Karkalas J. Resistance to acid hydrolysis of lipid complexed amylose and lipid free amylose in linterized waxy and non-waxy barley starch. Carbohydr Res. 1993 Jul; 245 (2): 289-302 [ Links ]
39. Chi H, Xu K, Wua X, Chen Q, Xue D, Song Ch, et al. Effect of acetylation on the properties of corn starch. Food Chem. 2008 Feb; 106 (3): 923-928. [ Links ]
40. López OV, García MA, Zaritzky NE. Film forming capacity of chemically modified corn starches. Carbohydr Polym. 2008 Sep; 73 (4): 573-581. [ Links ]
41. Varnam AH, Sutherland JP. Carne y productos cárnicos. Tecnología, Química y Microbiología. 1era ed. Zaragoza, España: Acribia, S.A; 1995. [ Links ]