Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.19 no.3 Medellín Seot./Dec. 2012
PHARMACEUTICAL CARE
VALIDATION OF A METHODOLOGY FOR INPATIENT PHARMACOTHERAPY FOLLOW-UP
VALIDACION DE UNA METODOLOGÍA PARA EL SEGUIMIENTO FARMACOTERAPEUTICO EN PACIENTE HOSPITALIZADO
Jesús BECERRA C. M.Sc.1*, Fernando MARTINEZ M. Ph.D.2, Martha BOHORQUEZ C. M.Sc.3, Martha L GUEVARA U. Q.F.1, Edgar RAMIREZ N. Q.F.1
1 Departamento de Farmacia. Universidad Nacional de Colombia. Carrera 30 No.45-03. Bogotá Colombia.
2 Facultad de Farmacia Universidad de Granada. Granada, España.
3 Departamento de Estadística. Universidad Nacional de Colombia.
* Autor a quien se debe dirigir la correspondencia: jbecerrac@unal.edu.co.
Received: 25 May 2012 Accepted: 09 September 2012
ABSTRACT
Background: Pharmacotherapy follow-up is a practice in which the pharmacist assumes responsibility for the patient's drug-related Problems. Its goal is to achieve positive clinical outcomes. Methods to perform pharmacotherapy follow-up have centered principally on ambulatory patients. Objective: The purpose of this study is to propose and validate a methodology for inpatient pharmacotherapy follow-up. Methods: A systematic review was performed. This consisted in a comprehensive search of databases containing studies published in English or Spanish during 1998 - 2008, and that sought to improve the transfer of accurate information about Pharmacotherapy follow-up in inpatients. The key terms used to conduct the search were identified in consultation with clinical experts and included: Pharmacotherapy follow-up methods, pharmacotherapy follow-up, drug therapy problems, and validation. A comparative table was elaborated to differentiate and evaluate the advantages of each of the proposed methodologies. The information gathered allowed to propose a sequence of general steps for inpatient Pharmacotherapy follow-up. To validate the methodology, a descriptive study was carried out with 32 randomly selected patients and was independently followed up by two pharmacists to assess the reproducibility of the process. Results: Pharmaceutical Care Practice: The Clinician's Guide, proposed by Cipolle & Strand. Applied Therapeutics: The Clinical Use of Drugs, the DÁDER method, and the IASER program, were selected. 79 drug therapy problems (DTPs) were identified and resolved, where errors in necessity of medication had the highest incidence (46.6%), followed by effectiveness (24.5%) and safety (28.9%). The degree of agreement among researchers in the identification and resolution of DTPs was quantified using the kappa coefficient, showing a high concordance (90% CI). The Fisher's exact test determined that DTPs are related to the duration of the follow up, number of medications, length of the stay and previous hospitalizations. Conclusions: The methodology allows identifying, preventing and resolving DTPs. It proved to be reproducible and have a high degree of concordance between applications.
Keywords: Pharmaceutical care, pharmacists, inpatient, validation.
RESUMEN
Antecedentes: El Seguimiento Farmacoterapéutico es la práctica profesional donde el farmacéutico asume la responsabilidad de la medicación del paciente con el objetivo de obtener resultados clínicos positivos. Los métodos actuales para realizar Seguimiento Farmacoterapéutico se han centrado principalmente en pacientes ambulatorios. Objetivos: Proponer y validar una metodología de Seguimiento Farmacoterapéutico para paciente hospitalizado. Métodos: Se realizó una revisión sistemática mediante la búsqueda exhaustiva en bases de datos de estudios publicados en inglés o español durante el periodo 1998-2008. La búsqueda se concentró en estudios que utilizaron metodologías de Seguimiento Farmacoterapéutico en las cuales se identificara, previniera y resolviera problemas de la medicación de un paciente hospitalizado. Los principales términos utilizados para llevar a cabo la búsqueda fueron identificados en consulta con expertos clínicos e incluyó: métodos o metodologías, Seguimiento Farmacoterapéutico, seguimiento farmacoterapéutico en pacientes hospitalizados, problemas relacionados con medicamentos y validación de metodologías. Se realizó una comparación de las mismas, para establecer las ventajas y desventajas y la factibilidad de su aplicación en el entorno hospitalario. Para validarla se adelantó un estudio descriptivo en 32 pacientes, de manera aleatoria, con características distintas, que fueron seguidos de manera independiente por dos farmacéuticos para evaluar la reproducibilidad del proceso. Resultados: Las metodologías de Cipolle y Strand, Pharmaceutical Care Practice: the Clinician's Guide, Applied Therapeutics: The Clinical Use of Drugs, el método Dader y el programa IASER, fueron seleccionadas. Se identificó y se resolvió 79 resultados negativos a la medicación (RNM), de necesidad (46,6%), efectividad (24,5%) y seguridad (28,9%). El grado de acuerdo entre investigadores en la identificación y resolución de RNMs se cuantificó con el coeficiente de concordancia kappa encontrando alto acuerdo (90% CI). La prueba Fisher relacionó características del paciente y cantidad de RNMs detectados. El tiempo de seguimiento, número de medicamentos, días de estancia y hospitalizaciones previas, tienen mayor relación. Conclusiones: La metodología diseñada permite identificar, prevenir y resolver RNMs, mostrando además ser reproducible y tener un alto grado de concordancia entre las aplicaciones.
Palabras clave: Atención Farmacéutica, farmacéutico, paciente hospitalizado, validación.
INTRODUCTION
Pharmaceutical Care is defined by Cipolle et al., 2004 (1), as ''a patient-centered practice in which the practitioner assumes responsibility for the patient's drug-related needs and is held accountable for this commitment''.
The goal of Pharmaceutical care is to procure positive clinical outcomes. Some of the desirable outcomes are: the cure of the patient's disease, elimination or amelioration of the patient's symptoms, arresting or slowing of a disease process, and preventing a disease or symptomatology. This, in turn, involves three major functions: to identify, resolve and prevent current and potential drug therapy problems (DTPs) (2, 3).
DTPs are undesirable events experienced by the patient that are related, or are suspected to be related, to the drug therapy and that interfere with the success of the drug therapy (4, 5).
DTPs can be classified into the following categories:
• Necessity: This category refers to the administration of unnecessary drug therapy (invalid medical indication of the drug therapy, multiple drug products are being used for a condition that requires a single drug, the medical condition is more appropriately treated with nondrug therapy, drug therapy is being taken to treat an avoidable adverse reaction associated with another medication, the problem is being caused by drug abuse, alcohol use, or smoking) and additional drug therapy (a medical condition related to not receiving a necessary medication, preventive drug therapy to reduce the risk of developing a new condition, a medical condition requires of additional pharmacotherapy to attain synergistic or additive effects).
• Effectiveness: Problems related to the inefficacy of the drug therapy (the drug is not the most effective for the medical problem being treated, the medical condition is refractory to other drug products, the dosage form of the drug product is inappropriate, and the drug product is not an effective product for the indication being treated) and low drug dosages (the dose is too low to produce the desired response, the administration interval is too infrequent to produce the desired response, a drug interaction is reducing the amount of active drug available, and the duration of the drug therapy is too short to produce the desired response).
• Safety: These include adverse drug events (ADEs; when the drug causes an undesirable reaction that is not dose-related, a safer drug product is required due to risk factors, a drug interaction causes an undesirable reaction that is not dose-related, the dosage regimen was administered or changed too rapidly, the drug product causes an allergic reaction, and the drug product is contraindicated due to risk factors) and problems related to high drug dosages (the dose is too high, the dosing frequency is too short, the duration of the drug therapy is too long, a drug interaction occurs resulting in a toxic reaction to the drug product, and the dose was administered too rapidly) (1, 6).
Studies have shown that the most frequent DTP is related to missing the administration of the proper dosage or not taking the drug at the proper time, a problem that could be easily detected by a pharmacist (7). About 19 - 80% of DTPs can be avoided or prevented (8, 9). ADEs such as taking the wrong medication or having an adverse reaction to the medication are reported in 11% of patients (10). Bates et al., 1995 (11), identified 247 ADEs and 194 potential ADEs, of which 1% had a fatal outcome and 42% could have been prevented.
In Ibero-America, Pharmacotherapy follow-up studies have focused on specific health services, a particular type of patient or disease, or on a certain group of drugs (12 - 14). This study proposes and validates a pharmacotherapy follow-up methodology that can be used to detect DTPs on inpatient patients and to validate the methodology procedure.
It is not possible to promote any pharmacist interventions as positive models for reducing medication errors and drug therapy problems. Insufficient research was undertaken with any particular type of intervention, and there were concerns regarding the level of evidence and quality of research (15).
MATERIALS AND METHODS
The study was carried out in two stages:
Stage 1: Assemblage of the proposed methodology
It included a systematic review of the literature. This consisted in a comprehensive search of databases containing studies published in English or Spanish during 1998 - 2008, and sought improving the transfer of accurate information about Pharmacotherapy follow-up on inpatients. The key terms used to conduct the search were identified in consultation with clinical experts and included: Pharmacotherapy follow-up methods, pharmacotherapy follow-up, drug therapy problems, and validation. One reviewer examined all titles and abstracts. The full articles of potentially relevant papers were obtained and each study was evaluated according to the following criteria: type of intervention, country and place where the study was carried out, inclusion and exclusion criteria, number of participants, and the proportion of evaluated patients. Once having evaluated the quality of the studies, the chosen methodologies were selected as a model, evaluating the steps proposed by each Pharmacotherapy follow-up methodology and its results.
A comparative table was elaborated to differentiate and evaluate the advantages of each of the proposed methodologies. The information gathered allowed to propose a sequence of general steps for inpatient Pharmacotherapy follow-up.
Stage 2: Validation of the proposed pharmacotherapy follow-up methodology
An observational descriptive study of a crosssectional nature was carried out in hospitalized elderly patients during 4 months (January - May 2008). Patients were chosen by simple random sampling without replacement. The inclusion criteria considered were males and females over the age of 60 who had multiple pathologies (≥3), were taking at least two or more drugs concurrently and that had been under hospital care for less than 24 h. Patients with a hospital stay of more than 24 h were excluded from the study.
The sample size was calculated taking into account the standard deviation of the age of the geriatric patients under hospital care during the last year, which was 10.3 years. Age information was supplied by the hospital's statistical office and the standard error was set at 3 years. The resulting sample size for a confidence level of 90% was 32 patients, according to equation 1:
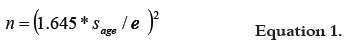
This stage encompassed the selection of patients: Among the patients admitted to the hospital database, those over the age of 70 who had been hospitalized for no more than 1 day were selected. Each patient's medical history was reviewed to check the number of prescribed drugs and comorbidities; only those patients who were being administered two or more drugs and had three or more comorbidities were classified as eligible and given a sequential number. Subsequently, the selection was randomized. Each researcher was in charge of the simultaneous Pharmacotherapy follow-up of four patients until patients were discharged. The selection process was repeated until gathering the entire sample of 32 patients, to which the proposed methodology was applied.
This stage focused on determining the capacity of the new methodology to identify, prevent and resolve DTPs. The sampled patients included inpatients with different characteristics to allow assessing the robustness of the methodology. Each patient received Pharmacotherapy followup from two pharmacists independently to assess the reproducibility of the methodology. The methodology was validated according to the kappa coefficient and its significance was determined at a 90% of confidence in order to establish whether the two researchers using the same methodology agreed in the detection of DTPs. Statistical procedures were carried out using the free software R. Additionally, the effects that characteristics such as gender, age, number of comorbidities, length of hospital stay, number of previous hospitalizations and prescribed medications may have on the appearance of DTPs were also analyzed. DTPs were classified into necessity, efficacy and safety. Finally, the average time required by a pharmacist to implement the methodology was estimated and the influence of this time on the results was also assessed.
RESULTS
A systematic review established that there are several Pharmacotherapy follow-up methodologies and programs. The most widely documented and applied Pharmacotherapy follow-up methods were used as reference to propose a new methodology. The mehtods were: pharmaceutical care Practice: The Clinician's Guide developed by Cipolle & Strand (1). Applied Therapeutics: The Clinical Use of Drugs, proposed by Young, Kradjan, Guglielmo, Alldredge, Corelli and Koda-Kimble, The DÁDER Pharmacotherapy follow-up Method developed by the Research Group on Pharmaceutical care, University of Granada, Spain and The Iaser Program, developed by the University of Valence (16 - 18).
The DADER method is useful for any type of patient, suffering any disease or health condition, in any environment, and applicable by any pharmacist. It was originally designed for community pharmacy and is currently used at other health care levels. In 2005, it was revised with two fundamental objectives: universalization and simplification, so it could be applied to inpatients. The adapted DÁDER method consists of 7 stages: service offering, first interview, assessment form, study stage, assessment stage, intervention stage and results of the intervention (19).
The Pharmaceutical Care Practice developed by Cipolle & Strand can be applied in all areas: community, hospital, long-term care, and clinic. It can be used to address all types of patients with all types of diseases and being administered with any type of drug treatment (1). The steps to follow up the patient's pharmacotherapy are: the diagnostic study of the pharmacotherapy, evaluation of the patient's medications, the needs related to the therapy in order to identify drug problems and their causes, developing care plans that include therapy goals and follow-up evaluation of results. All decisions of the Pharmacotherapy follow-up practice are documented (20).
The IASER© method is a standardized approach to Pharmaceutical Care, specialized in hospital care. It envisions five sequential and cyclical processes: identification of patients with improvement opportunities, pharmaceutical action, pharmacotherapy follow-up, (individual) evaluation, and publication of the results. It provides an operational outline with special focus on the health care process (18).
The SOAP method is a strategy for medical history analysis based on the health problems of the patient. It consists of 4 elements: subjective (S): it is important to take into account subjective information that other health care professionals include in the medical record; objective (O): it corresponds to the data registered in the medical history, such as different test results, procedures and evaluations; they can include vital signs, findings of the physical examinations, X-ray results, ECG, etc.; drugs are also considered as objective information; analysis (A): refers to the objective and subjective information that must be used to develop a therapeutic plan. The method has three main elements for the evaluation of each problem: etiology, revision of the recommended therapy and evaluation of the ongoing therapy and/ or new therapies; and finally, the plan (P): which considers all the recommendations obtained during the analysis, stipulates drug changes (inclusion or removal) and the strategies to be determined, goals to be achieved and the parameters that will allow continuing with the plan (16, 21).
Table 1 shows the most relevant aspects considered in the design of the proposition for a new Pharmacotherapy follow-up methodology. The aspects of the proposed methods are shown in Figure 1.
Patient characterization
The demographic characteristics of the patients were considered to analyze risks factors. For example, age to establish administration considerations, weight to individualize the dose, etc. The sample consisted of 62% males and 38% females. The average age of the patients was 81.7 years (range 70 - 93) and their average weight 62.6 kg. According to the reasons for the consultation, 50% of the patients complained of pain, angina and dysnea as their main health problems. After the number of hospitalizations was established through the hospital reports and an interview with the patient, it was found that 37.5% of the patients had been hospitalized in 3 previous occasions. The number of hospitalizations could not be established for 22% of the patients because they couldn't remember the exact number and no hospital records were available. The average time of hospitalization was less than 10 days for 90% of the patients.
Patients presented a total number of 98 illnesses. Of these, 34% were cardiopathies, 11.2% dyslipemia, and 10.2% pulmonary obstructive chronic disease (POCD; Asthma) and the remaining 44.6% corresponded to a variety of other illnesses, thus hindering a more detailed classification. The number of medications ranged from 3 to 25 per patient, with 81.25% of patients taking more than 9 drugs. Of these drugs, 52% could be grouped into one of the following five pharmacological groups: cytoprotective agents, painkillers, anti-hypersensitive drugs, anticoagulant drugs and antibiotics, with cytoprotective agents being the most prescribed medication (11.4%).
The identification and classification of DTPs has an important influence on the assistance that a patient receives. A total of 79 DTPs were identified and treated, not including undetected adverse drug interactions. DTs were classified according to the DÁDER method that DTPs related to receiving or not receiving a necessary medication were the most frequent ones (46.6%), followed by DTPs due to effectiveness (24.5%) and safety (28.9%).
Among DTPs related to necessity, 75% were due to receiving unnecessary drugs and 25% to untreated health conditions. In 73% of the DTPs associated to effectiveness, an inappropriate drug was administered and in 27% the administrated dosage was too low. All DTPs related to safety corresponded to adverse drug reactions.
Interventions can resolve the DTPs, prevent onset of new DTPs and satisfy the patient's needs. Although this was not an intervention study, for ethical considerations DTPs were treated as soon as they were detected. Prescription errors were the most common cause of intervention (28%).
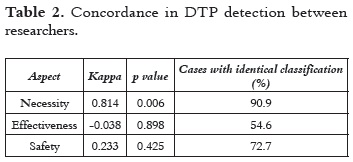
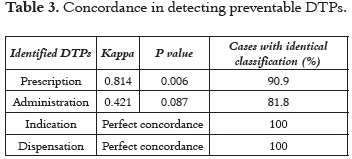
Information on the patient's pharmacological follow-up supplied by the two researchers who had similar training and experience was collected at the same time. The degree of agreement of the researchers on DTPs detection and resolution was quantified with the Kappa coefficient (κ) and subjected to the respective test of hypothesis, which resulted significant at a 90% confidence level (Table 2). In regards to the detection of DTPs related to necessity, the degree of concordance between researchers was almost perfect (see Table 2 and 3), which demonstrates the strength of the methodology for DTP identification (22).
Documenting DTPs simultaneously with all other health professionals allows pharmacists to act together with the patient and the attending doctors so as to prevent DTPs during hospitalization. The use of the proposed Pharmacotherapy follow-up methodology allowed researchers to detect preventable DTPs with a good degree of agreement (Table 4).
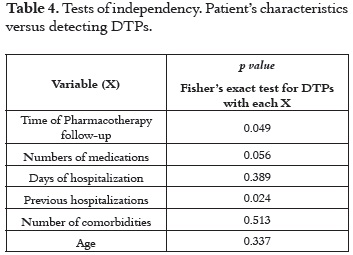
The results of the Fisher's exact test allowed determining whether there was a relationship between the patient's general characteristics and the number of DTPs being detected. As shown in (Table 4), the number of DTPs was significantly related to the length of time the patient was under Pharmacotherapy follow-up, the number of medication being administered, duration of the hospitalization and the number of previous hospitalizations.
The amount of previous hospitalizations is relevant in the onset of DTPs as it could be related to the presence of undiagnosed pathologies and therefore to reiterative hospitalization.
Finally, the detection of DTPs depended on the pharmacists skills and clinical experience, as shown by the significant correlation found between the time of pharmacotherapy follow-up and the patient's improvement; the longer the length of the pharmacotherapy follow-up is, the smaller the patient's risk to developing DTPs. The estimated average time necessary to conduct pharmacotherapy follow-up varied between 3.5 and 5 hours per patient, per day.
Ethnic differences in attitudes to medicines and medicines-taking are not apparent, although there are some commonalities in terms of needs of support and advice around medicines use (23).
DISCUSSION
A careful examination of the Pharmacotherapy follow-up methodologies selected based on the literature revision indicated that the Applied Therapeutics method: The Clinical Use of Drugs is not designed for pharmacists, but written for medical evaluation, as it contributes with an appropriate methodology for addressing SOAP clinical cases. This methodology was not considered for the design of the pharmacological follow-up methodology here presented. The method proposed by Cipolle & Strand and the DÁDER method share high similarity. The difference is that the DÁDER method describes the drug assessment and includes two activities, current situation and study phase, none of which is present in Cipolle & Strand's method. Moreover, it considers checking the patient's drug bag as an important activity to help in the pharmacological follow up. In Cipolle & Strand's method, the documentation of the intervention is unclear and could be understood that it is left to the discretion of the pharmacist what to do and how to act in the intervention, as no record of such is left.
The main difference between these two approaches lies in the DTP classification system. In Cipolle & Strand there are four categories: indication, effectiveness, safety and compliance, while in the DÁDER method there are three categories: indication, effectiveness and safety; adhesion is not included as it does not correspond to a clinical outcome but rather to a process.
The IASER method shows significant differences compared to other methods. It implements a quality assurance criterion to the practice of Pharmacotherapy follow-up, particularly in such a specialized environment as hospitals.
A careful revision of the three methodologies described above was done to design a pharmacotherapy follow-up methodology suitable for inpatients. The resulting methodology consists of 4 sequential and cyclic steps, which constitute the core of the Pharmacotherapy follow-up procedure: Identification and confirmation of DTPs, pharmaceutical intervention and evaluation of the results of the pharmaceutical intervention. It was necessary to design and adapt patient forms.
DTPs related to the need for new or additional drug therapy can be identified by comparing the patient's medication needs with the need for drug therapy. The pharmacist cannot detect DTPs by simply reviewing the list of drugs taken by a patient, i.e., it is not enough to focus on the medication, it is necessary to focus on patient as well. Pharmacological follow-up methodologies help to center pharmacy activities, which used to focus exclusively on the medication, now on the patient as well.
To identify the drug outcomes, additional information to the one provided in the medical history should be gathered by interviewing the patient directly. The quality of the drug information in the medical history is not good for this purpose, probably because it is built by professionals other than the pharmacist; this information is crucial because the effectiveness and safety of the pharmacotherapy is assessed based on it and on laboratory tests, diagnostic procedures, a physical exam, signs and symptoms. The identification of DTPs can be guaranteed by conducting a systematic and continuous follow up of the pharmacotherapy. The sporadic appearance of visible symptoms such as a rash, redness, and the like, provide a mechanism for identifying DTPs associated to drug safety.
Although it is best not to prolong a patient's stay in the hospital, investing more time to the pharmacological follow-up increases the probability of detecting DTPs; a process to which the pharmacist can contribute significantly.
CONCLUSIONS
The Pharmacotherapy follow-up methodology for inpatients described in this paper is reproducible, as indicated by the high degree of reproducibility found in the detection of DTPs between independent researchers applying the same methodology. Such concordance was not affected by demographics aspects or health conditions of the patients included in this study, which allow concluding that the methodology is robust for different types of patients.
This methodology is suitable for identifying, preventing and resolving DTPs, without restricting the health practitioner's freedom to achieve these goals through different mechanisms. Moreover, this DTP detection procedure is explicitly described so that it can be uniformly applied by pharmacists and taught to students interested in learning about pharmacotherapy follow-up.
REFERENCES
1. Cipolle RJ, Strand LM, Morley PC. Pharmaceutical care practice: the clinician's Guide. 2nd ed. United States of America: The McGraw-Hill Companies; 2004. 394 p. [ Links ]
2. Cobaugh DJ, Amin A, Bookwalter T, Williams M, Grunwald P, Lacivita C, Hawkins B. ASHP–SHM joint statement on hospitalist– pharmacist collaboration. Am J Health Syst Pharm. 2008 Feb 1; 65 (3): 260 - 263. [ Links ]
3. Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990 Mar; 47 (3): 533 - 543. [ Links ]
4. Van Mil JW, Westerlund LT, Hersberger KE, Schaefer MA. Drugrelated problem classification systems. Ann Pharmacother. 2004 May; 38 (5): 859 - 867. [ Links ]
5. Fernandez-Llimos F, Faus MJ, Gastelurrutia MA, Baena MI, Martinez-Martinez F. Evolución del concepto de problemas relacionados con medicamentos: resultados como el centro del nuevo paradigma. Seguimiento Farmacoterapéutico 2005; 3 (4): 167 - 188. [ Links ]
6. Comité de Consenso de Granada. Tercer Consenso de Granada, sobre Problemas Relacionados con Medicamentos (PRM) y Resultados Negativos de la Medicación (RNM). Ars Pharm. 2007; 48 (1): 5 - 17. [ Links ]
7. Crook M, Ajdukovic M, Angley C, Soulsby B, Doecke C, Stupans I, et al. Eliciting comprehensive medication histories in the emergency department: the role of the pharmacist. Pharmacy Practice. 2007 Abr-Jun; 5 (2): 78 - 84. [ Links ]
8. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug Reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998 Apr; 279 (15): 1200 - 1205. [ Links ]
9. Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1997 Jan; 277 (4): 301 - 306. [ Links ]
10. Eaton L. Adverse reactions to drugs increase. BMJ. 2002 Jan; 324 (7328): 8. [ Links ]
11. Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995 Jul; 274 (1): 29 - 34. [ Links ]
12. Silva M, Calleja M, Tuneu L, Fuentes B, Gutiérrez J, Faus MJ. Seguimiento del tratamiento farmacológico en pacientes ingresados en un servicio de cirugía. Farm Hosp. 2004 ; 28 (3): 154 - 169. [ Links ]
13. Fontana D, Soláuthurry N. Cuidado farmacéutico en pacientes pediátricos hospitalizados: adaptación de la metodología Dáder. Farm Hosp. 2003; 27 (2): 78 - 83. [ Links ]
14. Gil-Navarro MV, Bautista J, Santos B, Calleja MA, Marín-Gil R. Seguimiento farmacoterapéutico en pacientes hospitalizados en tratamiento con fentanilo transdérmico. Rev Soc Esp Dolor. 2006; 13 (4): 238 - 245. [ Links ]
15. Manias E, Williams A, Liew D. Interventions to reduce medication errors in adult intensive care: a systematic review. Br J Clin Pharmacol. 2012 Feb 20; 74 (3): 411 - 423. [ Links ]
16. Koda-Kimble MA, Young LY, Kradjan WA, Guglielmo BJ, Alldredge BK, Corelli RL. Applied Therapeutics: The Clinical Use of Drugs. 8th ed. Philadelphia: Lippincott Williams & Wilkins Publisher; 2006. Chapter 9, 10; 76. [ Links ]
17 Grupo de Investigación en Atención Farmacéutica, Universidad de Granada. Cuidado farmacéutico: Método Dáder. 3a rev. Pharmacy Practice. 2006; 4 (1): 44 - 53. [ Links ]
18. Climenti M, Jiménez N. Manual para la atención farmacéutica. 3ª ed. Valencia, España: AFAHPE. Hospital Universitario Dr Peset; 2005. 157 p. [ Links ]
19. Sabater HD, Silva-Castro MM, Faus MJ. Método Dáder: Guía de seguimiento Farmacoterapeutico. 3ª ed. Granada, España: Grupo de Investigación en Atención Farmacéutica (CTS-131). Universidad de Granada; 2007. 128 p. [ Links ]
20. Zierler-Brown S, Brown TR, Chen D, Blackburn RW. Clinical documentation for patient care: Models, concepts, and liability considerations for pharmacists. Am J Health Syst Pharm. 2007 Sep; 64 (17): 1851-1858. [ Links ]
21. Bhopal J. Simple soap system. Brit Med J. 1981 Oct; 283 (6296): 889 - 892. [ Links ]
22. Fisher, RA. Statistical Methods for Research Workers. 14th ed. CO: Hafner; 1970. 362p. [ Links ]
23. Bassett CD, Krass I, Bajorek B. Ethnic differences of medicinestaking in older adults: a cross cultural study in New Zealand. Int J Pharm Pract. 2012 Apr; 20 (2): 90 - 98. [ Links ]