Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Vitae
versão impressa ISSN 0121-4004
Vitae vol.19 no.3 Medellín set./dez. 2012
BIOTECHNOLOGY
EVALUATION OF THE INDUCTION OF LIPOLYTIC ENZYMES FROM A Pseudomona aeruginosa ISOLATED FROM AFRICAN PALM FRUIT (Elaeis guineensis)
EVALUACIÓN DE LA INDUCCIÓN DE ENZIMAS LIPOLÍTICAS A PARTIR DE UNA Pseudomona aeruginosa AISLADA DEL FRUTO DE PALMA AFRICANA (Elaeis guineensis)
Yomaira USCÁTEGUI1, Carlos JIMÉNEZ-JUNCA1, Camilo SUÁREZ2, Erlide PRIETO-CORREA1*
1 Facultad de Ingeniería. Universidad de La Sabana. Chía, Cundinamarca, Colombia.
2 Facultad de Minas. Universidad Nacional de Colombia. Medellín, Antioquia, Colombia.
* Autor a quien se debe dirigir la correspondencia: erlide.prieto@unisabana.edu.co.
Received: 03 Juny 2012 Accepted: 18 December 2012
ABSTRACT
Background: Extracellular lipases are found in the culture broth when the fermentation is at the end of the exponential phase. Lipases can be induced easily since they are produced by the presence of oily sources or other materials as surfactants, fatty acids, some esters, glycerol and biliary salts. Objective: The aim of this work is to study the effect of carbon source concentration and the use of inductors on biomass production, and the lipolytic activity of a bacterium isolated from mature palm oil fruits. Methods: The yield biomass/substrate was evaluated with glucose as carbon source at different concentrations (3, 5, 7, 10, 15 y 20 g/L) by dry weight and OD (600 nm). Lipolytic activity was evaluated by spectrophotometric assay using p-nitrofenilpalmitate at 37°C for 15 min. Results: Gram negative microorganisms with lipolytic activity isolated from palm fruit were identified as Pseudomona aeruginosa. The growth of the bacteria was inhibited when glucose was used at concentrations greater than 5%. The production of lipase was induced by using three inducers (Palm oil, Tween 20 and palm oil:Tween 20 mixture), at three different induction times (0, 11 and 18 hours of fermentation). The highest activity (3,81 μmoles/ mL*min) was observed when the palm oil:Tween 20 mixture was added at 11 hours of fermentation. The kinetic of p-nitrophenylpalmitate hydrolysis using the supernatant of a culture induced with palm oil:Tween 20 mixture at 11 hours showed the production of p-nitrophenol beyond 300 minutes, with the greatest hydrolysis rate during the first 7 minutes. Conclusions: The growth of P. aeruginosa was not affected by using glucose as carbon source at concentrations of 3% and 5%. There was a basal level of lipase production without inducer, and greater lipolytic activity was achieved with the addition of inducers.
Keywords: Lipolytic activity, fatty acids, enzyme induction, hydrolysis.
RESUMEN
Antecedentes: Las lipasas extracelulares se encuentran en los medios de cultivo cuando las células alcanzan el final de la fase exponencial de crecimiento. Las lipasas son fácilmente inducibles, es así como se producen en presencia de fuentes lipídicas u otros materiales como surfactantes, ácidos grasos, algunos ésteres, sales biliares y glicerol. Objetivo: El objetivo de este trabajo es estudiar el efecto de la concentración de fuente de carbono y el uso de inductores sobre la producción de biomasa, y la actividad lipolítica de una bacteria aislada de frutos maduros de palma de aceite. Métodos: Se determinó el rendimiento de biomasa/sustrato utilizando como fuente de carbono glucosa en diferentes concentraciones (3, 5, 7, 10, 15 y 20 g/L) mediante la técnica de peso seco y densidad óptica (600 nm). La actividad lipolítica fue evaluada con p-nitrofenil palmitato a 37°C por 15 min. Los productos de la reacción se determinaron espectrofotométricamente (410 nm). Resultados: Los microrganismos Gram negativos con actividad lipolítica fueron identificados como Pseudomona aeruginosa. El crecimiento de las bacterias fue inhibido cuando la glucosa se utilizó a concentraciones superiores a 5%. La lipasa fue inducida usando tres inductores (aceite de palma, Tween 20 y la mezcla de aceite de palma:Tween 20), en tres tiempos diferentes de inducción (0, 11 y 18 horas de fermentación). La actividad más alta (3,81 µmoles/mL*min) se observó cuando se añadió la mezcla de aceite de palma:Tween 20 a las 11 horas de fermentación. La cinética de la hidrólisis de p-nitrofenil palmitato utilizando el sobrenadante de un cultivo inducido con la mezcla de aceite de palma:Tween 20 a las 11 horas presentó producción de p-nitrofenol hasta más allá de 300 minutos, con la mayor tasa de hidrólisis durante los primeros 7 minutos. Conclusiones: El crecimiento de P. aeruginosa no se vio afectada utilizando glucosa como fuente de carbono en concentraciones de 3% y 5%. Se encontró un nivel basal de producción de lipasa sin inductor y se obtuvo una mayor actividad lipolítica con la adición de inductores.
Palabras clave: actividad lipolítica, ácidos grasos, inducción enzimática, hidrólisis.
INTRODUCTION
Lipases are lipolytic enzymes, classified as carboxylic ester hydrolases (EC. 3.1.1.3) which break ester bounds of acylglycerides by adding a water molecule generating free fatty acids and glycerol. Lipases are versatile and interesting in biotechnology since they catalyze different kind of reactions such as partial or complete hydrolysis of triacylglycerols, along with the esterification, transesterification and interesterification of lipids (1 - 3).
Lipases are used as additives in food, fine chemicals, detergent, waste water treatment, cosmetics, pharmaceuticals, leather processing and biomedical assays. Furthermore, lipases have an important application in the field of bioenergy, especially in biodiesel production, which is an expanding sector, as a result of the worldwide rising demand on the use of renewable energy (3).
Lipases have been found in many species of animals, plants, and microorganisms. A wide range of microorganisms (bacteria, fungi and yeast) can produce lipases with different enzymological properties and substrate specificities. The enzymes from microbial sources are receiving more attention because of their interesting characteristics. Some bacteria produce and excrete lipase that can catalyze the hydrolysis and synthesis of long chain acylglycerol, which can be produced with high regioselectivity, enantioselectivity, under mild conditions, while keeping the stability in organic solvents, and so forth. Amongst bacteria, Achromobacter, Alcaligenes, Arthrobacter, Pseudomonas, Staphylococcus, Chromobacterium spp. and Serratia sp. have been exploited for the production of lipases (3 - 5).
The production of microbial lipolytic enzymes is influenced by nutritional and physical-chemical factors. Nutritional factors like carbon and nitrogen sources and the presence of lipids in the culture medium have been studied to improve the enzyme production from different types of microorganisms (6 - 8). Vegetable oils such as soybean, corn, olive, and sunflower, non-metabolizable polysaccharides and carbohydrates have been used as carbon sources (2, 6). The use of different nitrogen sources (peptone, yeast extract, corn liquor, ammonium sulfate, ammonium nitrate) at different concentrations and combinations have shown effects on lipolytic enzymes production (6, 9). On the other hand, when non fatty substances are used as carbon sources the presence of lipids (butter, olive oil, canola oil, fish oil) in culture medium influence the lipolytic activity and production of microbial lipases (10, 11).
Since lipases have diverse properties in accordance with their origin it is not possible to generalize about the favorable conditions to get a great lipolytic activity or high production, so it is necessary to study each source to find the suitable conditions of production (4).
The aim of this work is to study the effect of carbon source concentration and the use of inductors on biomass production, and the lipolytic activity of a bacterium isolated from mature palm oil fruits.
MATERIALS AND METHODS
Microorganism
A gram negative microorganism with lipolytic activity isolated previously from palm fruit oil in this laboratory was used to produce the lipase (12). The stock cultures of the strain were maintained in glycerol (0.5% v/v) at -80 °C. The microorganism was identified with an API® 20 NE - 24 to 48-hour identification of Gram negative non-Enterobacteriaceae characterization kit. The test results were analyzed through apiwebTM software identification.
Culture medium and carbon source
Synthetic medium (MM) was used as production medium, which contained (NH4)2SO4 (0.5% w/v), casein peptone (1.0% w/v), K2HPO4 (0.5% w/v), and MgSO4*7H2O (0.1% w/v). The initial pH of the medium was adjusted to 7.5 with sodium phosphate buffer 50 mM prior to sterilization. Glucose at concentrations of 3%, 5%, 10%, 15% and 20% (w/v) was used as carbon source in the fermentation broth (MM).
Pre-inoculum and fermentation conditions
Pre-inoculum was prepared by transferring one loopful of cryopreservation culture to a liquid medium consisting of a nutrient broth Tryptic Soy Broth (TSB) (30 g/L) and allowed to grow on a rotary shaker with shaking at 200 rpm and at 37°C for 18 h. This was used as an inoculum which contained approximately 106 cells per mL. For lipase production, conical flasks (100 mL) containing 20 mL MM medium were inoculated with 1% inoculum and incubated on a rotary shaker at 200 rpm and 37 °C for 24 h (13 - 15).
Biomass determination
Cell biomass was determined by measurement of the absorbance of cells at 600 nm, after being centrifuged at 13000g for 20 min and 4°C, washed twice with saline solution 0.85% (w/v) and re-suspended in saline. Dry cell weight was measured by filtering a 25 mL sample through a 0.2 um Millipore membrane, followed by washing with 50 mL distillated water and drying at 60°C to constant weight. A standard curve (10 OD corresponds to 17.4 g/L dry weight) was used to determine the biomass production during the fermentation.
Effect of lipid and surfactant on lipase production
Several experiments were carried out to determine the inducing effect by adding 0.3 g/L of an oil and a surfactant, namely, crude palm oil, Tween 20 and a Tween 20:Palm oil mixture (4.8:1 v/v). The inducers were added at different times of growth (0, 11 and 18 h) in order to determine the effect of addition time. Samples were taken during the stationary phase after 24 h of growth.
Lipase assays
At the end of fermentation process, the flasks were taken and harvested to measure the lipase activity in the fermented broth: samples were centrifuged at 10,000 rpm for 20 minutes at 4°C and the supernatant was taken to the lipase assay. The spectrophotometric lipase assay was used to determine the lipolytic activity (16). The substrate solution was prepared by mixing 0.5 mL of 100 mM of pNPP in acetonitrile with 2 mL of ethanol and 47.5 mL of phosphate buffer (pH 7.5). The assay mixture consisted of 1.5 mL of substrate solution and 0.5 mL of cell-free supernatant. The assay mixture was incubated at 37°C for 15 min and the p-nitrophenol released was measured at 405 nm in Spectronic-117 spectrophotometer against a control without supernatant. One unit of enzyme is defined as the amount of enzyme liberating 1 μmol of pnitrophenol mL-1 min-1 under the assay conditions.
RESULTS
Bacterial Strain
The biochemical identification that test showed the isolated Gram negative microorganism with lipolytic activity belongs to the genus and species Pseudomonas aeruginosa in a 99.9% accuracy.
Effect of the carbon source concentration
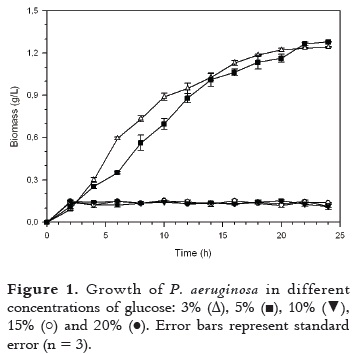
Since there is a relationship between the biomass concentration and lipase production (11, 17), the effect of the carbon source (glucose) concentration was studied. Figure 1 shows the evolution of the P. aeruginosa cultures.
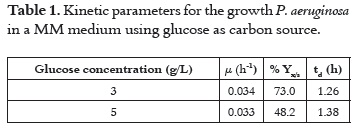
The greatest growth of P. aeruginosa was observed when glucose concentration was low (3 and 5 g/L), while the growth was minimum at higher concentrations. Specific growth rate (µ) (Table 1) was similar for concentrations of 3 and 5 g/L glucose but the yield biomass/substrate (Yx/s) (at 24 h) was greater for 3 g/L. It is also noted that a concentration of 3 g/L the doubling time (td) was lower, so the fermentation is more efficient since the bacteria grow rapidly when the doubling time is low. A concentration of 3 g/L of glucose is used in the rest of this work.
Effect of induction agents
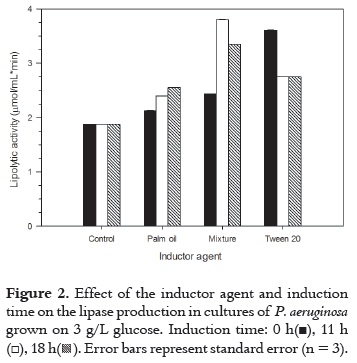
When P. aeruginosa was grown using 3 g/L of glucose as carbon source with the inductors added at different times during the fermentation process the lipolytic activity showed distinct values (Figure 2).
Control sample shows P. aeruginosa produced lipase (1.88 µmol/mL*min) when it grew only in glucose (without inductor) and the production was greater when inductors were added at different times. The greatest lipolytic activity (3.81 µmol/ mL*min) was observed with palm oil:Tween 20 mixture added at 11 hours. Greater lipolytic activity was obtained with Tween 20 and the mixture, rather than with palm oil. The addition of the palm oil:Tween 20 induced the highest lipase activity at each time, except at 0 h, when the activity was the greatest by using Tween 20 as inductor agent. There was an increase of lipolytic activity with the induction time when palm oil was added: 2.13, 2.39 and 2.55 µmol/mL*min at 0, 11 and 18 h respectively; however, there was not a clear tendency when other inductors were used.
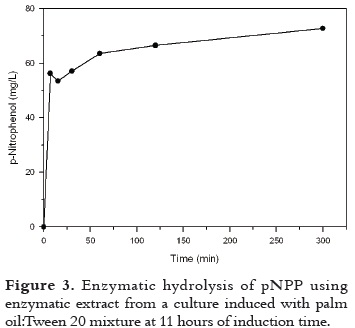
Kinetic of enzymatic hydrolysis (Figure 3) was determined for palm oil: Tween 20 mixture at 11 h of induction time, which presented the greatest lipolytic activity. It is clear from the figure that there was lipolytic activity beyond 300 min since p-nitrophenol was still produced. A great hydrolysis rate was observed during the first 7 minutes when production of p-nitrophenol increased from 0 to 56.2 mg/L. The production of p-nitrophenol was increasing until 300 min at lower rates until a constant rate tendency is observed.
DISCUSSION
Effect of carbon source concentration
Under the fermentation conditions assayed, bacteria growth in different concentrations of carbon source was evaluated. As observed in Figure 1 there was a cell growth inhibition at higher glucose concentration (above 5 g/L). The concentrations of glucose that favor the obtention of an acceptable growth of P. aeruginosa were 3% and 5%. This result agrees with Ito et al., 2001(13), who found that cell growth of Pseudomonas aeruginosa LST-03 was affected by both glucose and ammonium concentration. In the same way, Takac et al., 2008 (18), stated that high concentrations of glucose suppress the lipolytic activity.
On the other hand, Beyenal et al., 2003 (19), and Gupta et al., 2004 (6), pointed out that glucose in small amounts is necessary for the initial growth phase of microorganisms for the releasing of extracellular lipase.
The combination of two types of carbon sources (carbohydrate and oil) does not always improve lipase production. For example Nahas, 1987 (20), working with R. oligosporus, and Rapp, 1995 (21), working with Fusarium used a mixture of glucose and oleic acid as carbon-source, and it was found that the consumption was produced in a sequential pattern, so the lipase production was repressed by the glucose present in the defined medium.
Effect of induction agents
For lipase production different lipid based carbon sources have been reported as inducer as well as carbon source (alone or in combination with carbohydrates) (1, 10, 18, 22). Results obtained from this study showed that there was lipolytic activity in the control sample without inducer agent. This is known as basal level of the enzyme and it allows concluding that glucose, as carbon source at low concentrations (less than 5 g/L), does not repress the release of the enzyme.
As shown in Figure 2 the inducer agents stimulated the release of enzymes, since all media containing inducer agents showed values greater than 1,88 µmol/mL*min, which was the value for the control sample.
The addition of a surfactant (Tween 20) as inductor improved the lipolytic activity regarding with control sample at the three induction times. This result agrees with the studies of Gilbert et al. 1991 (23), Nahas, 1987 (20), and Dominguez et al., 2003 (24), who used Tween 20, Tween 80 and Tween 100, finding improvements on lipase production, although the results depended on the type of Tween used. Moreover, Grasian et al., 2008 (5), stated that the addition of any surfactant induces the lipase production. Deive et al., 2009 (11), explains the effect of surfactants on the improvement of lipase production by the solubilization of the lipids on the membrane, forming micelles and extracting membrane proteins (intrinsic and peripheral proteins) which are the responsible proteins of lipolytic activity in membrane bound of microorganisms.
According to Wu et al., 2004 (25), higher levels of lipase production were observed when the substrate formed an emulsion, thereby presenting an interfacial area to the enzyme. This has resemblance with the earlier P. pseudoalkaligens F-111 lipase, where addition of Triton-X-100 increased the alkaline lipase production by 50-fold (26). An elaborated study was performed by Lin et al., 1995, (26) about the effect of Triton-X-100 on lipase production. Triton-X-100 may directly act as a positive activator of lipase gene or may induce the expression of lipase gene by removing the repressor molecules. They also inferred that Triton-X-100 mechanistically promotes both uptake and exit of compounds from the cell through modification of plasma membrane permeability. Contrastingly, the studies on lipase production by Staphylococcus sp. indicated the positive influence of Tween 80 (27).
Favorable levels of lipolytic enzyme production were found in this work when the mixture (Palm oil:Tween 20) was used as inductor agent at 11 hours of growth during the exponential phase. According to Illanes et al., 2008 (4), and Reis et al., 2010 (28), lipases catalyze the hydrolysis of lipidic substrates when they are in micelles, small aggregates or particles in emulsion, because the place where lipolysis occurs requires at least two phases and a larger contact area. On the other hand, Bañó et al., 2003 (29), and Reis et al., 2009 (30), argue that using an emulsion facilitates the interfacial activation of lipases features, as the emulsion facilitates substrate access to enzyme active site.
Bisht et al., 2012 (31), investigated the suitability of adding different carbon sources for lipase production by a mutant strain. Supplementation of production medium with starch tremendously boosted the lipase activity (about 129.3%) as compared to control. Similar results were reported by Pogaku et al., 2010 (27), while investigating the lipase production by Staphylococcus sp. Lp12. Rathi et al., 2001 (32), also reported increase in lipase production from Burkholderia cepacia in the presence of oil and glucose as sugar additive.
We can use this fact to conclude, that mixture is the best inducer agent compared with the other two, because the activity increment may be due to the presence of more interfacial area, facilitating interaction between enzyme and substrate. Another possibility could be that higher enzyme release was due to the combined effect between palm oil and Tween 20, because the latter one can act separately and facilitate the release of more lipase.
According to previous results, the mixture showed the best results, and for this reason the kinetic of enzymatic hydrolysis was determined with this mixture as shown in Figure 3. The mixture (palm oil:Tween 20) was added to the culture medium at 11 hours of fermentation.
The release of 100 µM of p-NP was achieved at 300 minutes of reaction with p-NPP when inducer was added at 11 hours of fermentation; this means that when the bacteria was in the exponential phase it was found a greater rate of reaction.
CONCLUSIONS
According to the results, it can be stated that the effect of adding inductors to the fermentation medium increases the levels of enzyme production. Similarly, it was observed that using glucose as carbon source does not suppress the basal level release of the enzyme in the fermentation medium.
Hydrolysis of p-NPP as substrate was determined by the release of p-nitrophenol, acting as an indicator of the presence the enzyme in the medium after 15 minutes of reaction. The reaction showed that an inducting agent stimulated the release of the enzyme into the medium, and the best yield was obtained with mixture (palm oil:Tween 20) as inducer. The mixture was added at 11 hours of fermentation medium and the value was 3,81 µmoles/ mL*min for fermentation medium.
A preliminary kinetic study of enzymatic hydrolysis was performed for the best inducer agent (mixture) found. The reaction conditions were: 100 µM of p-NPP, 300 minutes of reaction, and the inductor was added at 11 hours of fermentation medium. The kinetic of enzymatic hydrolysis for the control sample has lower values than those achieved when the emulsion was used as an inducer in the release of the enzyme.
ACKNOWLEDGEMENTS
To Colciencias and the Universidad de La Sabana for financing the research project ''Obtención de enzimas lipolíticas a partir de microorganismos aislados del fruto de palma aceitera'', which is part of this work.
REFERENCES
1. Toscano L, Gochev V, Montero G, Stoytcheva M. Enhanced production of extracellular lipase by novel mutant strain of Aspergillus niger. Biotechnol Biotech Eq. 2011 Feb; 25 (1): 2243 - 2247. [ Links ]
2. Treichel H, Oliveira D, Mazutti M, Luccio M, Oliveira JV. A Review on Microbial Lipases Production. Food Bioprocess Tech. 2010 Apr; 3 (2): 182 - 196. [ Links ]
3. Salihu A, Alam MZ, AbdulKarim MI, Salleh HM. Lipase production: An insight in the utilization of renewable agricultural residues. Resour Conserv Recycling. 2012 Jan; 58 (0): 36 - 44. [ Links ]
4. Illanes A, Álvarez L, Álvaro G. Esterificación quimioselectiva de fitosteroles de madera mediante lipasas. Rev Col Biotecnol. 2008 Jul; 10 (1): 17 - 35. [ Links ]
5. Grasian I, Palanichamy E, Austin J, Palanisamy I, Arunachalam P. Investigation of Lipase Production by Milk Isolate Serratia rubidaea. Food Technol Biotechnol. 2008 Jan-Mar; 46 (1): 60 - 65. [ Links ]
6. Gupta R, Gupta N, Rathi P. Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol. 2004 Jun; 64 (6): 763 - 781. [ Links ]
7. Hasan F, Shah AA, Hameed A. Methods for detection and characterization of lipases: A comprehensive review. Biotechnol Adv. 2009 Nov-Dic; 27 (6): 782 - 798. [ Links ]
8. Li N, Zong M. Lipases from the genus Penicillium: Production, purification, characterization and applications. J Molec Catal B. 2010 Sep; 66 (1-2): 43 - 54. [ Links ]
9. Sharma R, Chisti Y, Banerjee UC. Production, purification, characterization, and applications of lipases. Biotechnol Adv. 2001 Dec; 19 (8): 627 - 635. [ Links ]
10. Gupta N, Sahai V, Gupta R. Alkaline lipase from a novel strain Burkholderia multivorans: Statistical medium optimization and production in a bioreactor. Process Biochem. 2007 Apr; 42 (4): 518 - 526. [ Links ]
11. Deive FJ, Carvalho E, Pastrana L, Rúa ML, Longo MA, Sanroman MA. Strategies for improving extracellular lipolytic enzyme production by Thermus thermophilus HB27. Bioresour Technol. 2009 Jul; 100 (14): 3630 - 3637. [ Links ]
12. Peña F. Aprovechamiento de aceites residuales del proceso de fritura como sustrato para el desarrollo de microorganismos productores de lipasas (Proyecto de grado Ingeniero de Producción Agroindustrial). Chía, Colombia: Facultad de Ingeniería Universidad de la Sabana; 2006. [ Links ]
13. Ito T, Kikuta H, Nagamori E, Honda H, Ogino H, Ishikawa H, et al. Lipase production in two-step fed-batch culture of organic solvent-tolerant Pseudomonas aeruginosa LST-03. J Biosci Bioeng. 2001; 91 (3): 245 - 250. [ Links ]
14. Gaoa X, Cao S, Zhang K. Production, properties and application to nonaqueous enzymatic catalysis of lipase from a newly isolated Pseudomonas strain. Enzyme Microb Technol. 2000 Jul 1; 27 (1-2): 74 - 82. [ Links ]
15. Kanwar L, Goswami P. Isolation of a Pseudomonas lipase produced in pure hydrocarbon substrate and its application in the synthesis of isoamyl acetate using membrane-immobilised lipase. Enzyme Microb Technol. 2002 Nov 1; 31 (6): 727 - 735. [ Links ]
16. Gupta N, Rathi P, Gupta R. Simplified para-nitrophenyl palmitate assay for lipases and esterases. Anal Biochem. 2002 Dec 1; 311 (1): 98 - 99. [ Links ]
17. Lee D, Koh Y, Kim K, Kim B, Choi H, Kim D, et al. Isolation and characterization of a thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS Microbiol Lett. 1999 Oct 15; 179 (2): 393 - 400. [ Links ]
18. Takac S, Marul B. Effects of lipidic carbon sources on the extracellular lipolytic activity of a newly isolated strain of Bacillus subtilis. J Ind Microbiol Biotechnol. 2008 Sep; 35 (9): 1019 - 1025. [ Links ]
19. Beyenal H, Chen SN, Lewandowski Z. The double substrate growth kinetics of Pseudomonas aeruginosa. Enzyme Microb Technol. 2003 Jan 2; 32 (1) : 92 - 98. [ Links ]
20. Nahas E. Control of Lipase Production by Rhizopus oligosporus under Various Growth Conditions. J Gen Microbiol. 1987; 134 (1): 227 - 233. [ Links ]
21. Rapp P. Production, regulation, and some properties of lipase activity from Fusarium oxysporum f. sp. vasinfectum. Enzyme Microb Technol. 1995 Sep; 17 (9): 832 - 838. [ Links ]
22. Yadav K, Adsul M, Bastawde K, Jadhav D, Thulasiram H, Gokhale D. Differential induction, purification and characterization of cold active lipase from Yarrowia lipolytica NCIM 3639. Bioresour Technol. 2011 Nov; 102 (22): 10663 - 10670. [ Links ]
23. Gilbert E, Cornish A, Jones C. Purification and properties of extracellular lipase from Pseudomonas aeruginosa EF2. J Gen Microbiol. 1991 Sep; 137 (9): 2223 - 2229. [ Links ]
24. Domínguez A, Costas M, Longo MA, Sanromán A. A novel application of solid state culture: production of lipases by Yarrowia lipolytica. Biotechnol Lett. 2003 Aug; 25 (15): 1225 - 1229. [ Links ]
25. Wu HS, Tsai MJ. Kinetics of tributyrin hydrolysis by lipase. Enz Microb Technol. 2004 Dec; 35 (6-7): 488 - 493. [ Links ]
26. Lin SF, Chioul CM, Tsaiz YC. Effect of Triton-X-100 on alkaline lipase production by pseudomonas pseudoatcatigenes F-111. Biotechnol Lett. 1995; 17: 959 - 962. [ Links ]
27. Pogaku P, Suresh A, Srinivaslu P, Reddy SA. Optimization of lipase production by Staphylococcus sp. Lp12. Afr J Biotechnol. 2010 Feb 8; 9 (6): 882 - 886. [ Links ]
28. Reis P, Watzke H, Leser M, Holmberg K, Miller R. Interfacial mechanism of lipolysis as self-regulated process. Biophys Chem. 2010 Apr;147 (3): 93 - 103. [ Links ]
29. Bañó M, Gozález H, Abad C. Long-chain fatty acyl-CoA esters induce lipase activation in absence of a water-lipid interface. Biochim Biophys Acta. 2003 Jun 10; 1632 (1-3): 55 - 61. [ Links ]
30. Reis P, Holmberg K, Miller R, Leser M, Raab T, Watzke H. Lipase reaction at interfaces as self-limiting processes. C R Chimie. 2009 Jan 2; 12 (1): 163 - 170. [ Links ]
31. Bisht D, Kumar K, Singh N. Enhanced Production of Extracellular Alkaline Lipase by an Improved Strain of Pseudomonas aeruginosa MTCC 10,055. Am J Applied Sci. 2012; 9 (2): 158 - 167. [ Links ]
32. Rathi P, Saxena R, Gupta R. A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem. 2001 Oct; 37: 187 - 192. [ Links ]