Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.19 no.3 Medellín Seot./Dec. 2012
BIOTECHNOLOGY
LACTIC ACID PRODUCTION VIA CASSAVA-FLOURHYDROLYSATE FERMENTATION
PRODUCCIÓN DE ÁCIDO LÁCTICO VIA FERMENTATIVA A PARTIR DE HIDROLIZADO DE HARINA DE YUCA
Joan E. QUINTERO M. Ing.1, Alejandro ACOSTA C. M.Sc.2, Carlos MEJÍA G M.Sc.3, Rigoberto RÍOS E. Ph.D.4, Ana M.TORRES L. M.Sc.5*
1 Estudiante de Maestría en Ingeniería Química del Grupo Biotransformación. Escuela de Microbiología, Universidad de Antioquia. Medellín, Colombia.
2 Profesor ocasional e Investigador Grupo Biotransformación. Escuela de Microbiología. Universidad de Antioquia. Medellín, Colombia.
3 Profesor vinculado y Coordinador del Grupo Biotransformación. Escuela de Microbiología. Bioprocesos. Departamento de Ingeniería Química. Universidad de Antioquia. Medellín, Colombia.
4 Profesor vinculado e Investigador Grupo Bioprocesos. Departamento de Ingeniería Química. Facultad de Ingeniería. Universidad de Antioquia. Medellín, Colombia.
5 Profesor ocasional e Investigador Grupo Bioprocesos. Programa de Bioingeniería. Universidad de Antioquia. Medellín, Colombia.
* Autor a quien se debe dirigir la correspondencia: atorres@udea.edu.co.
Received: 16 March 2012 Accepted: 17 December 2012
ABSTRACT
Background: Lactic acid (LA) is a carboxylic acid widely used as preservative, acidulant, and/or flavouring in food industry; it is also used as a raw material for the production of lactate ester, propylene glycol, 2,3-pentanedione, propanoic acid, acrylic acid and acetaldehyde. In recent years, the demand for LA production has dramatically increased due to its application as a monomer for poly-lactic acid synthesis, a biodegradable polymer used as a plastic in many industrial applications. LA can be produced either by fermentation or chemical synthesis; the former route has received considerable interest, due to environmental concerns and the limited nature of petrochemical feedstocks; thus, 90% of LA produced worldwide is obtained by fermentation, this process comprises the bioconversion of a sugar solution (carbohydrates) into LA in the presence of a microorganism. Objectives: This work is aimed at studying the effect of pH control and culture media composition on the LA production using renewable sources from the agroindustry sector. Methods: A Lactobacillus brevis strain is used to perform lab scale experiments under aerobic and anaerobic conditions, using three different culture media compositions: a high nutritional content medium (MRS), as a reference, a low nutritional content medium with glucose as the only carbon source (GM), and a potential low nutritional content medium with cassava flour as carbon source (HY1). Results: The higher LA production is accomplished under anaerobic conditions, 17.6 ± 0.1, 12.6 ± 0.2 y 13.6 ± 0.2 g LA/L, for MRS, GM and HY1 medium, respectively. The effect of pH on LA biosynthesis in a 5L bioreactor is also studied using the HY1 medium. For a fermentation time of 120 h, the highest LA concentration obtained was 24.3 ± 0.7g LA/L, productivity 0.20 g/L/h, YP/S 0.32g LA/g syrup, at pH 6.5. Conclusions: These results are comparable with those using expensive carbon sources such as glucose, and show cassava flour as a promising low-cost substrate source for lab and eventually large scale LA biosynthesis.
Keywords: Lactic acid, cassava flour, cassava waste material, Lactobacillus brevis, pH effect.
RESUMEN
Antecedentes: El ácido láctico (AL) es un ácido carboxílico utilizado en la industria alimentaria como conservante, acidulante y saborizante; también es usado como materia prima para la producción de éster de lactato, propilenglicol, 2,3-pentanodiona, ácido propanoico, ácido acrílico y acetaldehído. La demanda de AL ha aumentado debido a su aplicación como monómero en la síntesis de ácido poli-láctico, un polímero biodegradable usado como plástico en aplicaciones industriales. El AL puede ser producido por fermentación o síntesis química; la primera ruta ha recibido mayor interés, debido a las preocupaciones ambientales y a la limitación en materias primas petroquímicas. El 90% del AL producido en el mundo se obtiene por fermentación, la cual involucra la bioconversión de una solución de azúcar en AL, en presencia de un microorganismo. Objetivos: En este trabajo se evalúa el efecto del pH y de medios de cultivos sobre la producción de AL a partir del cultivo de Lactobacillus brevis, usando fuentes renovables provenientes del sector agroindustrial. Métodos: El desarrollo experimental a escala de laboratorio considera la evaluación de tres medios de cultivo: uno de alto contenido nutricional (MRS), medio de referencia, uno de medio contenido nutricional, con glucosa como única fuente de carbono (GM), y un medio de cultivo de bajo contenido nutricional, con jarabe de yuca como fuente de carbono (HY1). Resultados: La más alta producción de AL se obtiene bajo condiciones anaeróbicas, 17,6 ± 0,1, 12,6 ± 0,2 y 13,6 ± 0.2 g AL/L, para los medios MRS, GM y HY1, respectivamente. El trabajo contempla el estudio del efecto del pH sobre la biosíntesis de AL en reactor de 5L, usando el medio de cultivo HY1. Para 120h de cultivo la más alta concentración de AL que se obtiene es 24,3 ± 0,7g AL/L, productividad 0,20 g/L/h, y un rendimiento de sustrato en producto (Y P/S ) de 0,32g AL/g jarabe, a pH 6,5. Conclusiones: Estos resultados son comparables con los obtenidos en otros trabajos usando glucosa como fuente de carbono, y permiten considerar al jarabe de yuca como un potencial sustrato de bajo costo y alta disponibilidad para la producción de AL a escala de laboratorio, y eventualmente a escala industrial.
Palabras clave: Ácido láctico, jarabe de yuca, sustratos económicos, Lactobacillus brevis, efecto del pH.
INTRODUCTION
Organic acids production has become a valuable and economic alternative to chemical synthesis, thus contributing the biotechnological world market with carboxylic acids, enols, sulfonic acids, mercapto-compounds, and phosphonic acids.
Biotechnological processes use renewable resources, such as silage, grains, syrups, molasses, and cheese whey as feedstock. Moreover, the products from fermentation have a higher safety degree, which is significant to human health. Some organic acids cannot or are difficult to be produced via chemical synthesis. Taking lactic acid as an example, the chemical synthesis produces a racemic mixture of lactic acid (L and D-forms) while fermentation can selectively synthesize the desired stereospecific lactic acid (1).
Among others, the major applications for organic acids have traditionally been food, beverage, pharmaceuticals, cosmetics, detergents, plastics and resin industry. Organic acids are considered important as starting materials, mainly due to their characteristic functional groups; though for some organic acids the actual market is relatively small, the novelty of economical production processes would create new markets by providing new opportunities for the chemical industry (2). Lactic acid (LA) was the first organic acid to be produced at industrial scale in 1880. Recently, LA demand has dramatically increased for poly-lactic acid production, where it is used as a monomer (3, 4).
The organic acid production has been traditionally carried out by solid-state fermentation, surface fermentation and submerged fermentation (5-7). The organisms traditionally used in fermentation processes are gram-positive bacteria belonging to the species Lactobacillus, Carnobacterium, Leuconostoc, Tetragenococus, Pediococcus, Streptococcus, Lactococcus, Vagococcus, Esterococcus, Aerococcus and Weissellas (8, 9). For a lactic acid strain to be considered as prominent it is required to strength its metabolic capabilities, to rapidly and completely convert cheap raw materials into LA with minimal nutritional requirements, and also providing high yields of the preferred stereoisomer without by-product formation (10).
Polymer producers, and industrial users in general, commonly require large quantities of relatively low-cost LA. Therefore, raw materials for LA production should be cheap, have low levels of contaminants, be able to induce the synthesis of few or no by-product formation, have ability to be fermented with little or no pretreatment, and have year-round availability. An alternative for LA production would be the use of refined materials. Nonetheless, the economical balance is unfavorable since refined substrates are extremely expensive, causing even higher production costs. Hence, research aiming to screen for cheaper raw materials for economic LA production is a very active field (10). In this regard, diverse sources such as soybean (11), potato (12), wood (13), corn liquor (14), and molasses have been studied. Among these, starchy (mainly sweet sorghum, wheat, corn, cassava, potato, rice and barley), and cellulosic materials, are widely preferred due to their low price, abundance and renewable characteristics (10). Despite the number of nitrogenous materials (whey permeate, yeast extract, malt sprouts, grass extract, peptones, beef extract, casein hydrolysate with supplementation of vitamins) that have been used to supplement the carbohydrate source for fast and heavy growth, yeast extract seems to be the most effective supplement (15).
For experimental studies, LA production is commonly carried out in erlenmeyers or lab scale batch bioreactors. Environmental conditions are usually set at 30 - 42ºC, 120 - 200 rpm, and pH ranging from 5 to 6.8 (16). Regarding the operation mode for LA production, batch, fed-batch, repeated batch, and continuous fermentations are the most frequently used. Commonly, higher LA concentrations are obtained in batch and fed-batch cultures, whereas a continuous process may render in a better productivity. Combining either batch or continuous processes with cell-recycling brings on even higher cell concentration and productivity (12).
This work aims at testing the use of cassava syrup as a potential unique carbon source for high-yield LA biosynthesis in a lab scale bioreactor, using Lactobacillus brevis. The study did also consider the important effect of pH on LA production by controlling pH culture conditions throughout the entire fermentative process.
Materials and Methods
Microorganism and culture medium
A Lactobacillus brevis strain was used. Cryogenic vials with MRS medium (in g/L): glucose 100, peptone 10, yeast extract 10, meat extract 10, K2HPO4 2, sodium acetate 5, ammonium citrate 2, MgSO4.7H2O 0.2, MnSO4.H2O 0.05 and 30% glycerol, were used for strain conservation at -4°C. Chemicals for MRS medium were from MERCK® (Frankfurt, Germany). The strain was maintained in Petri dishes with MRS medium and 1% agar at 4°C, and subcultured every other month.
For flask assays, inoculum preparation was carried out using MRS medium with a modified glucose concentration at 10 g/L. Inoculums for bioreactor operation were grown in the HY medium (in g/L): reducing sugars from enzymatic hydrolysis of cassava flour 30, yeast extract 15, KH2PO4 5.6, K2HPO4 4.16; initial pH set at 5.5. Operating conditions for inoculums were set at 38°C and 150 rpm for 24 h. For enzymatic hydrolysis of cassava, 0.13 g/L of CaCl were added to a 30% cassava solution. pH was adjusted at 5.5. Afterwards, 0.5 mL/L of Thermamil were added; temperature and stirring were set at 80°C and 500 rpm, respectively. After cooling to 50°C, and adjusting pH at 4.5, 1.5 mL/L of amyloglucosidase was added and the solution heated until 60°C, stirred at 150 rpm during 6h. After filtration, a 242 g/L solution of reducing sugars was obtained, having 76% of glucose (17).
In addition to the MRS medium, two culture media for LA production were used for flasks assays, the GM medium (in g/L): glucose 100, yeast extract 15, KH2PO4 5.6, K2HPO4 4.16, and the HY1 medium: reducing sugars from enzymatic hydroxylation of cassava flour 100, yeast extract 15, KH2PO4 5.6, K2HPO4 4.16; initial pH was set at 5.5. For bioreactor operation, the HY1 medium was used. Yeast extract was from OXOID® (Hampshire, England); glucose, KH2PO4 and K2HPO4 were from MERCK® (Frankfurt, Germany).
Lactic Acid production
Cultivation procedure
Aerobic and anaerobic culture conditions were used for studying the strain ability for LA production. The MRS, GM and HY1 media were tested for the production of LA in flasks of 100 mL with 50 mL of culture medium operating at 38°C, 150 rpm for 72h. For studying LA biosynthesis under anaerobic conditions, a low flow rate nitrogen stream was fed during 2 min after inoculation. Flasks were immediately sealed using rubber stoppers to ensure that cultures were not oxygen-contaminated.
In order to study the pH effect on LA biosynthesis, a 7.5L bioreactor BioFlo/CelliGen 115-New Brunswick® (Enfield CT, EEUU) was used. Operating conditions were set at 38ºC, 200 rpm for 76h. Inoculum preparation consisted of 490 mL HY. For pH control NaOH 2N and H3PO4 8.5% solutions were used. The pH effect on LA biosynthesis was studied at three levels: 4.5, 5.5 and 6.5. Results were compared with those from experiments carried out without pH control. In addition to LA production and pH level on-line determination, reducing sugar content was also measured.
Analytical Methods
Determination of reducing sugar was performed by the DNS method, as described elsewhere (18). LA was measured by HPLC AGILENT TECNHNOLOGY® (EEUU) using a C-610H column with 7.8 mm ID and 30 cm length; H3PO4 at 0.1% and 0.5ml/min as the mobile phase, UV detection at 210 nm, and 30°C (19).
Statistical Analysis
Assays for LA production were run in triplicate. For studying the effect of pH on LA biosynthesis the experiments in a 5L bioreactor were run in duplicate, as well. Thus, a total of three or two values per set of experiments were used to calculate mean and variance for each data point. Substrate and product concentrations were expressed as the mean value ± standard deviation.
RESULTS
Lactic Acid Production under aerobic and anaerobic conditions
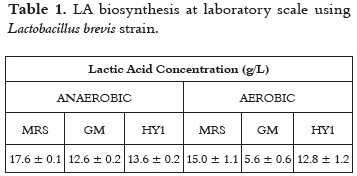
For the purpose of this work, a Lactobacillus brevis strain was used; table 1 shows values for LA production. Although the highest LA content was achieved under anaerobic conditions, statistical significance (P < 0.05) was only found for the GM culture medium, when comparing the effect of presence or absence of oxygen in the same medium. Similarly, statistical significance was found when comparing anaerobic LA production in the three evaluated culture media.
Based on these results and taking into account the nutritional requirements of Lactobacillus strains, the medium HY1, with cassava flour as carbon source, was selected for further experiments at bioreactor scale.
pH control effect on LA biosynthesis
LA biosynthesis is strongly influenced by pH control. According to Adamberg et al., 2003 (20), pH levels should not exceed values around 4-7. LA biosynthesis at bioreactor scale with no pH control, rendered a poor yield, YP/S = 0.24, productivity: 0.12 g/L.h and a low concentration of LA 9.55 ± 1.50 g/L, compared to that of erlenmeyer, 13.60 ± 0.20 g/L at the same culture conditions (medium HY1, T = 38°C, initial pH: 5.6). The differences between these results are, eventually, not only a scale difference effect but also the effect of a modified composition in the medium for inoculum preparation, using a rich medium (MRS) at erlenmeyer scale, and a poorer medium (HY1) at reactor scale.
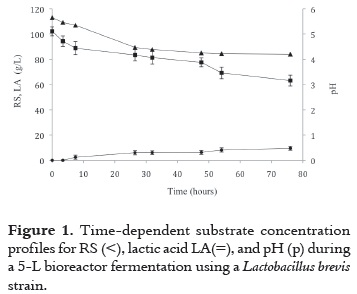
As it is observed in figure 1, while substrate is consumed at the beginning of the fermentation, LA biosynthesis takes 5h to start being accumulated, perhaps due to the lag period that the organism needs to get comfortable with the new environment. After 48h of cultivation, pH stabilizes around 4.2.
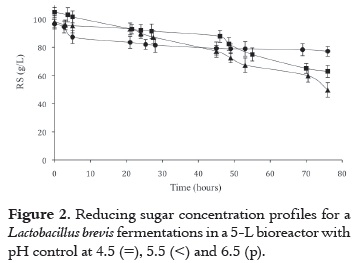
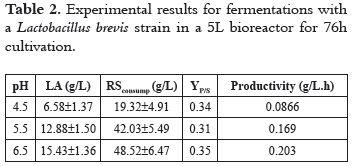
Figures 2 and 3 disclose the time variation for substrate and LA concentration, in fermentations carried out in a 5L biorreactor with pH control. As it is shown, the higher the pH, the higher the LA accumulation and a corresponding lower residual substrate concentration; thus, for pH controlled at 4.5, 5.5 and 6.5, LA production reached concentrations of 6.58 ± 1.37, 12.88 ± 1.50 and 15.43 ± 1.36 g/L, respectively.
After running a t-student test, it was found that there was no statistical significance (P > 0.05) by comparing LA concentration reached after a fermentation process with pH control at 5.5 and 6.5. Conversely, lower pH conditions (4.5) did result in a reduction of LA accumulation; both, pH values of 4.5 and 5.5, and, 4.5 and 6.5 did render a statistical significance (P < 0.05).
Table 2 summarizes the changes in product biosynthesis and reducing sugar (RS) consumption for different pH control levels. As it is observed, there is a major shift in LA accumulation when pH control is raised from 4.5 to 5.5, producing a nearly threefold higher LA concentration.
Due to pH control in the fermentation process, at pH 6.5, LA biosynthesis increased 61.6%, and YP/S and productivity were 45.4% and 69%, respectively, higher than those with no pH control.
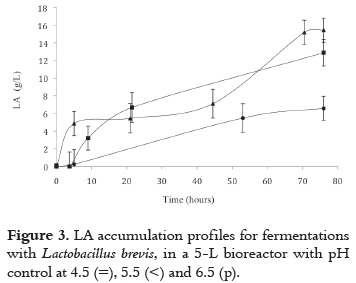
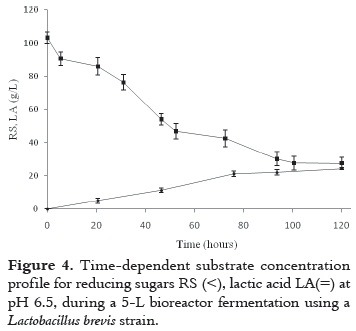
According to figure 3, LA accumulation profiles still show product biosynthesis activity at the 76th hour of cultivation; this might be the result of a scarce inoculum concentration and/or insufficient fermentation time for total substrate consumption. Therefore, in order to asses this hypothesis further experimentation was undertaken, increasing inoculum concentration in the HY culture medium in a 50%, and incubated at 28°C for 24h. Afterward, the 5L reactor was inoculated and the fermentation was extended until the 120th hour with pH controlled at 6.5. See figure 4.
LA accumulated mostly during the first 76 hours of cultivation, with no significant increment between the 76th and 120th hour. The largest LA accumulation, reached at 120h, was 24.3 ± 0.7 g/L; however, the concentration of LA obtained at the 76th hour with no inoculum concentration increment (15.43 ± 1.36 g/L), was lower than that reached at the same time of cultivation with increased inoculum concentration (21.23 ± 1.67 g/L); therefore, the improvement in LA biosynthesis was clearly the result of increasing inoculum concentration rather than enlarging time of cultivation.
DISCUSSION
The genus Lactobacillus has been widely known as the major LA producer strain. These organisms have limited ability for synthesizing their own growth factors, mainly B vitamins and aminoacids. Typically, they require carbon and nitrogen sources and diverse elements in the form of carbohydrates, aminoacids, vitamins and minerals. In addition, the use of assortments of aminoacids, peptides and amides, stimulate growth of lactic acid bacteria (LAB) yielding much higher values than those obtained with free aminoacids. LAB growth is also influenced by fatty acid and phosphate which are the most important salt in the LA fermentation. For most lactic strains, ammonium cannot serve as the only nitrogen source; yet, they apparently have shown some influence on amino acid metabolism. Conversely, the amount of minerals found in commercial complex media seems to be sufficient for LA metabolism (10).
Taking into consideration all the aforementioned, it can be stated that cassava f lour, the main component for the HY1 medium, became an appropriate substrate for LA biosynthesis. According to Cardona et al., 2011 (21), cassava flour has 81.48% of starch. When 300g/L of cassava f lour are prepared, 99% of starch is hydrolyzed reaching a glucose content of about 76%; the remaining part is maltodextrines. Based on this analysis, and besides reducing sugars, cassava flour has different ions including sodium, calcium, iron and magnesium, aminoacids and proteins that might have favored bacterial growth and product biosynthesis.
Even though Lactobacillus is a facultative anaerobic organism, its metabolic activity is enhanced in the absence of oxygen, among other factors, by the need of the oxidized form of the cofactor NAD+, which is reduced during the catabolic activity. The NADH donates its extra electrons to the pyruvate molecule formed during glycolysis; since the NADH has lost electrons, NAD+ regenerates and is again available for glycolysis (16). Yet, as a facultative anaerobic bacterium, Lactobacillus is able to ferment and also experience cellular respiration when oxygen is present; the process is called heterofermentative LA biosynthesis; in such a case, carbon dioxide and ethanol are present, thus rendering a LA yield reduction (22).
Conversely, though LAB is acid-tolerant, it has been shown that the pH is an important factor in acid fermentation including lactic acid (23). Different pH values in the medium did influence not only the cell growth but the biosynthesis rate. Moreover, variability in the medium pH might have stimulated a metabolic shift, a theme that merits further experimental evidence.
LA concentration reached in this study is comparable with most literature reports. For substrates such as glucose, corn flour, sawdust, molasses, whey, among others, LA production ranges from 1 to 100 g/L, using commercial strains such as Lb. plantarum, Lb. casei, Lb. delbrueckii, Lb. helveticus, among others (16). Siebold et al., 1995 (14), and Kious et al., 2000 (24), did report LA production close to 26.8 - 27.8 g/L and 13.3 - 19.6 g/L, respectively, using glucose as the unique substrate. The present study reports production of LA close to 24.3 ± 0.7g/L using an inexpensive substrate with low salt content, and what is most relevant, with a non-commercial strain.
CONCLUSIONS
It was demonstrated that LA biosynthesis can be accomplished competitively using alternative low-cost nutrient-rich substrates such as cassava flour. This kind of substrates show high nutrient and reducing sugar content as well as calcium and aminoacids, all of them available for the fermentative process.
Since Lactobacillus is able to ferment and also experience cellular respiration, in the presence of oxygen, carbon dioxide and ethanol were also synthesized, thus provoking a LA titer reduction; therefore, the largest LA accumulation was attained under anaerobic conditions. LA titers, reached in this study, were comparable to most literature reports, even those using refined and substantially more expensive substrates as unique carbon source.
LA biosynthesis was also dependant on pH control, since a strict control of pH level close to 6.5 rendered a 65% increment in LA accumulation, weighed against lower pH values. The larger LA accumulation might be the result of a metabolic shift at pH values above 6. This argument entails more detailed experimental support; work currently in progress.
AKNOWLEDGMENT
The authors thank the Comité para el desarrollo de la investigación – CODI Universidad de Antioquia for financial aid: Project MC07-01-13.
REFERENCES
1. De Lima C, Coelho F, Contiero J. The Use of Response Surface Methodology in Optimization of Lactic Acid Production: Focus on Medium Supplementation, Temperature and pH Control. Food Technol Biotechnol. 2010; 48 (2): 175 - 181. [ Links ]
2. Sauer M, Porro D, Mattanovich D, Branduardi P. Microbial production of organic acids: expanding the markets. Trends Biotechnol. 2008 Feb; 26(2):100-108. [ Links ]
3. Mohamed A, Yukihiro T, Kenji S. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J Biotechnol. 2010 Dec 20; 156 (4): 286 - 301. [ Links ]
4. Harington T, Hossain Md. Extraction of lactic acid into sunflower oil and its recovery into an aqueous solution. Department of Chemical and Materials Engineering. 2008 Jan 5; 218: 287 - 296. [ Links ]
5. López C, Zuluaga A, Herrera S, Ruiz A, Medina V. Producción de ácido cítrico con Aspergillus niger NRRL 2270 a partir de suero de leche. Rev Dyna. 2006 Nov; 73 (150): 39 - 57. [ Links ]
6. Gil R, Domínguez R, Pacho J. Bioproducción de ácido láctico a partir de residuos de cáscara de naranja: Procesos de separación y purificación. Tecnolog Ciencia. 2008; 23 (2): 79 - 90. [ Links ]
7. Darouneh E, Alavi A, Vosoughi M, Arjmand M, Sifkordi A, Rabaji R. Citric acid production: Surface culture versus submerged culture. African Journal of Microbiology Research. 2009 Sep; 3 (9): 541 - 545. [ Links ]
8. Sneath P, Bergey's. Manual of systematic bacteriology. Williams and Wilkins Baltimore. London. 1984; Vol. II: 464. [ Links ]
9. Hofvendahl K, Hhanhägerdal H. Factors affecting the fermentative lactic acid production from renowable resource. Enzyme Microb Tech. 2000 Feb 1; 26 (2-4): 87 - 107. [ Links ]
10. Young-Jung Wee, Jin-Nam Kim, Hwa-Won Ryu. Biotechnological Production of Lactic Acid and Its Recent Applications. Food Technol Biotechnol. 2006; 44 (2): 163 - 172. [ Links ]
11. Kwon S, Lee P, Lee E, Chang Y, Chang N. Production of lactic acid by Lactobacillus rhamnosus with vitamin-supplemented soybean hydrolysate. Enzyme Microbiol Tech. 2000 Feb 1; 26 (2-4): 209 - 215. [ Links ]
12. Anuradha R, Suresh A, Venkatesh K. Simultaneous saccharification and fermentation of starch to lactic acid. Process Biochem. 1999; 35: 367 - 375. [ Links ]
13. Moldes A, Alonso J, Parajó J. Resin selection and single-step production and recovery of lactic acid from pretreated wood. Appl Biochem Biotechnol. 2001 Aug; 95 (2): 69 - 81. [ Links ]
14. Siebold M, Frieling P, Joppien R, Rindf leisch D, Schügerl K, Röper H. Comparison of the production of lactic acid by three different lactobacilli and its recovery by extraction and electrodialysis. Process Biochem. 1995; 30 (1): 81 - 95. [ Links ]
15. Narayanan N, Roychoudhury P, Srivastava A. L (+) lactic acid fermentation and its product polymerization. Electro J Biotechn. 2004 Aug 15; 7 (2): 167 - 179. [ Links ]
16. Serna L, Rodríguez A. Producción Biotecnológica de ácido láctio: Estado del arte. Cienc Tecnol Aliment. 2005; 5 (1): 54 - 65. [ Links ]
17. Rios O, Castaño H. Escalado del proceso de producción de jarabes glucosados en un reactor Batch de 5 a 500 litros como etapa previa a la fermentación etanólica a partir de harina de yuca [Tesis de pregrado]. [Medellín, Colombia]: Universidad de Antioquia: 2009. 48p. [ Links ]
18. Breuil C, Saddler J. Comparisons of 3,5-dinitosalicylic acid and nelson–somogyi methods of reducing sugars and determining cellulose activity. Enzyme Microb Technol. 1984; 7: 327 - 332. [ Links ]
19. Hussain M, Rouch D, Britz M. Biochemistry of non-starter lactic acid bacteria isolate Lactobacillus casei GCRL163: Production of metabolites by stationary-phase cultures. Int Dairy J. 2009; 19 (1): 12 - 21. [ Links ]
20. Adamberg K, Kask S, Laht T, Paalme T. The effect of temperature and pH on the growth of lactic acid bacteria: a pH-auxostat study. Int J Food Microbiol. 2003 Aug 15; 85 (1-2): 171 - 183. [ Links ]
21. Cardona M, Agudelo L. Producción de Biopolímeros (Polihidroxialcanoatos) a partir de una cepa comercial empleando sustratos no convencionales [Tesis Maestría en Ingeniería]. [Medellín, Colombia]: Universidad de Antioquia; 2011. 50p. [ Links ]
22. Thomas T, Ellwood D, Longyear V. Change from homo- and heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr; 138 (1): 109 - 117. [ Links ]
23. Silva E, Yang S. Kinetics and stability of a fibrous-bed bioreactor for continuous production of lactic from unsupplemented acid whey. J Biotechnol. 1995 Jul 15; 41 (1): 59 – 70. [ Links ]
24. Kious J. Lactobacillus and lactic acid production [Tesis]. [Golden, Colorado]: Le Tourneau University; 2000. 32 - 33 p. [ Links ]