Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.20 no.1 Medellín Jan./Apr. 2013
NATURAL PRODUCTS
CHEMICAL COMPOSITION AND ANTI-IRRITANT CAPACITY OF WHOLE BODY EXTRACTS OF Ulomoides dermestoides (COLEOPTERA, TENEBRIONIDAE)
COMPOSICIÓN QUÍMICA Y CAPACIDAD ANTI-IRRITANTE DE EXTRACTOS DE CUERPO ENTERO DE Ulomoides dermestoides (COLEOPTERA, TENEBRIONIDAE)
Dary L. MENDOZA M.1*, Stephanie SAAVEDRA A.2
1 Programa de Química. Facultad de Ciencias Básicas. Universidad del Atlántico. Barranquilla, Colombia. dary_mendoza@yahoo.com.
2 Facultad de Ciencias Básicas. Universidad del Atlántico. Km. 7 antigua carretera a Puerto Colombia. Barranquilla, Colombia.
Received: 23 July 2012
Accepted: 10 April 2013
ABSTRACT
Background: In traditional medicine of Central and South America, the tenebrionid beetle Ulomoides dermestoides is used as an a phrodisiac, for the treatment of inflammatory diseases and cancer. Recently was reported cytotoxic and genotoxic properties of non-polar extract of U. dermestoides; also anti-inflammatory and immunomodulatory activity of aqueous whole body extract of beetle was reported, it suggests the existence of components with potential pharmacology use. On the other hand, it is necessary to identify those polar and non-polar extracts of U. dermestoides with anti-irritant properties for the membranes and blood vessels, which will be used in subsequence biological test and clinical assays. Objectives: The purpose of this research was to identify the chemical composition of methanolic and hexanic extracts of U. dermestoides, and to assess their anti-irritant capacity. Methods: The extracts were obtained from adult beetles of U. dermestoides. The chemical composition of the extracts was determined by gas chromatography-mass spectrometry (GC-MS) and the anti-irritant effect of each extract was evaluated by means of a modified assay of irritation of the chorioallantoic membrane (CAM) of fertilized chicken eggs (HET-CAM); the results were expressed as irritation index (IR). Results: Six common compounds were identified in both extracts: limonene, myristic, palmitic, estearic, oleic, and linoleic fatty acids. But in the alone methanolic extract were found: 1-pentadecanol, alpha-pinene, beta-phellandrene and alpha-terpinene, whereas in the hexanic extract were found: 2-methyl-p-benzoquinone, 2,4-dihidroxy-1-ethylbenzene, 2,5-dimethylquinone, saturated and unsaturated hydrocarbons and alcohols. The methanolic extract of U. dermestoides showed potential anti-irritant effect in the HET-CAM test (IR = 3.09 ± 0.11), similar to that observed with Nimesulida (IR = 2.05 ± 0.14), a non-steroid anti-inflammatory drug (NSAID) used as positive control for irritation inhibition reaction. The hexanic extract did not show anti-irritant capacity. Conclusions: The results demonstrated the anti-irritant effect of the methanolic extracts of U. dermestoides that could be attributed to compounds with anti-inflammatory activity as oleic acid and limonene.
Keywords: Ulomoides dermestoides, beetles, metabolites, biological assay, Chorioallantoic Membrane.
RESUMEN
Antecedentes: En medicina tradicional de Centro y Sur América, el escarabajo tenebrionido Ulomoides dermestoides es usado como afrodisíaco, en tratamiento de enfermedades inflamatorias y cáncer. Recientemente se reportó las propiedades citotóxicas y genotóxicas de un extracto no polar de U. dermestoides; también la actividad anti-inflamatoria e inmunomoduladora de un extracto acuoso del cuerpo entero del coleóptero, lo cual sugiere la presencia de compuestos con potencial uso farmacológico. Adicionalmente, se requiere identificar extractos polares y no polares del U. dermestoides con propiedades anti-irritantes para las membranas y vasos sanguíneos, los cuales serían usados en subsiguientes ensayos biológicos y en pruebas clínicas. Objetivos: El propósito de esta investigación fue determinar la composición química de extractos metanólicos y hexánicos del cuerpo entero de U. dermestoides, y evaluar su capacidad antiirritante. Métodos: Los extractos fueron obtenidos de coleópteros adultos de U. dermestoides. La composición química de los extractos fue determinada por cromatografía de gases acoplada a espectrometría de masas (CG-EM) y el efecto anti-irritante fue evaluado mediante ensayo modificado de irritación de la membrana corioalantoidea (CAM) de huevos fertilizados de gallina (HET-CAM); los resultados fueron expresados como índice de irritación (IR). Resultados: Se identificó 6 compuestos comunes en ambos extractos: limoneno, los ácidos grasos mirístico, palmítico, esteárico, oleico y linoleico. En el extracto metanólico también se encontró: 1-pentadecanol, alfa- pineno, beta-felandreno y alfa-terpineno; en el extracto hexánico: 2-metil-p-benzoquinona, 2,4-dihidroxi-1-etilbenzeno, 2,5-dimetil-quinona, hidrocarburos saturados e insaturados y alcoholes. El extracto metanólico mostró efecto anti-irritante potencial en el ensayo HET-CAM (IR = 3,09 ± 0,11), similar al observado con el fármaco Nimesulida (IR = 2,05 ± 0,14), un anti-inflamatorio no esteroide (AINES) usado como control positivo de la inhibición de la irritación. El extracto hexánico no mostró capacidad anti-irritante. Conclusiones: Los resultados demostraron el efecto anti-irritante de los extractos metanólicos de U. dermestoides, lo que podría atribuirse a compuestos con actividad anti-inflamatoria como el ácido oleico y el limoneno.
Palabras clave: Ulomoides dermestoides, escarabajos, metabolitos, bioensayo, membrana corioalantóide.
INTRODUCTION
Ulomoides dermestoides (Chevrolat, 1893) (synonyms: Martianers dermestoides; Palembus dermestoides), is an Asian tenebrionid beetle commonly known as the ''peanut beetle'' because it is a pest to peanuts and other grains (1). The beetles are eaten alive as an aphrodisiac in Southeast Asia (2, 3). In Central and South America it is used in the treatment of various illnesses such as bronchial asthma, dermatitis, rheumatoid arthritis, hemorrhoids, inflammations and pain in the liver and kidneys, Parkinson disease, diabetes mellitus, and different types of cancer (4-7).
Although the use of beetles in folk medicine is spread, few studies have been published about the insect compounds responsible for the potential healing effects. A recent publication has described the cytotoxic and genotoxic properties of U. dermestoides benzoquinone (1, 4-benzoquinones) on human lung carcinoma epithelial cell line A549; similar results were reported with the dichloromethane whole body extract, which could explain the positive results reported in alternative treatments for cancer (8). Another publication has reported the anti-inflammatory properties of polar whole body extract of U. dermestoides using carrageenan-induced paw edema assay in rats and human peripheral blood mononuclear cells (9); however, the specific anti-inflammatory compounds in this extract are unknown.
In addition, an in vitro study about crude extracts of the defense secretion of beetle Palembus ocularis reported inhibitory activity of the enzyme 5-lipoxygenase, suggesting a possible bronchodilator effect of these beetles, the same study showed anti-inflammatory effect from a polar extracts of defense secretion and their major compound, hydroquinone, using a modified in vivo test on the chorioallantoic membrane of the fertilized hen's egg (HET-CAM), which suggests that hydroquinone in the defense secretion of beetles have antiinflammatory properties (10).
HET-CAM test is a current method that allows determining anti-inflammatory activity and toxic effects of complex extracts, and has proven be useful in screening natural products (11). Irritation causes alterations in the vascular system of the CAM that result in membrane discoloration, hemorrhaging and increased perfusion. In the HET-CAM test the anti-inf lammatory activity occurred when irritation of the CAM, induced by an irritant agent, decreased and the blood vessel net appeared unchangeable (12, 13).
In this study we have analyzed the chemical composition of the methanolic and hexanic extracts of U. dermestoides and have determined their potential anti-irritant effect using HET-CAM test.
MATERIALS AND METHODS
Extracts
Specimens of Ulomoides dermestoides were identified in the Entomology Department of the Natural Sciences Institute (Universidad Nacional, Bogotá, Colombia, collection code ICN-45905). The specimens were kept protected from light and under controlled conditions for temperature (27 ± 2°C) and relative humidity (70-75%) and were only fed with wheat bran and whole-grain bread. Extracts were prepared from 2 g of adult beetles obtained from the culture and frozen at -70°C. To obtain methanolic extract, the protocol in Unruh et al., 1998 (14), was followed with modifications. Briefly, beetles were crushed using traditional friction fragmentation methods with a mortar under a current of liquid nitrogen, followed by extraction with 25 mL of methanolic solution (methanol 10% v/v, HCl 10mM and ascorbic acid 25 mM). Hexanic extract was obtained from fragmented beetles using 25 mL of hexane during 12 hours and under constant stirring. Both extracts were clarified by using centrifugal force at 3500 x g for 15 min at 4°C in a Beckman 81783 GS 6R centrifuge, and filtered through fiberglass membranes. All extracts were kept sealed and frozen until they were used.
GC-MS
Simultaneous extraction and concentration were done to the compounds found in the vapor phase of the methanolic extract by using headspace solid-phase microextraction (HS-SPME). A 65-μm PDMS/DVB fiber was used, and split injection was conducted using the mode with a volume of injection of 1 μL. The hexanic extract (1 μL) was injected manually. Chromatographic analysis of both extracts was performed in an Agilent Technologies 6890 Plus gas chromatograph coupled to an Agilent Technologies 5973 mass selective detector operated at a full scan of radio frequencies. A J & W Scientific DB - 5MS column (5%- phenyl- poly (dimethylsiloxane), 60 m x 0,25 mm x 0,25 μm). The compounds in each sample were identified using mass spectrometry data from the NIST MS Search Program, version 2.0.
HET-CAM Test
For this procedure, the chorioallantoic membrane (CAM) of fertilized chicken eggs of the Hi Line Brown was used. The CAM that surrounds the developing embryo is highly vascular, and it is considered insensitive to pain (15). Eggs were donated by the company ACONDESA, S.A. The procedures undertaken on the eggs were realized according to the provisions of Law 84 of 1989 and resolution 8430 of 1993 of Ministerio de Salud de Colombia, regarding biomedical research on animals.
Test preparation
The eggs were placed for eight days at 37.5°C in an incubator containing an automatic revolving mechanism. After that, the eggs were removed from the incubator and the position of the air sacs was marked with a pencil. Unfertilized eggs or eggs containing embryos were discarded. The remaining eggs were weighed, and only those weighing 60 ± 5 g were used in the HET-CAM test.
Samples and solutions
Test samples were the methanolic and hexanic extracts of U. dermestoides. Because hexane is irritating for the CAM, this solvent was evaporated of the hexanic extract using a HEIDOLPH 4000-G1 rotary evaporator, and the liquid residue was applied on the CAM; the methanolic extract was used directly in the anti-irritant test. An aqueous solution of sodium dodecyl sulphate (SDS 0.5% w/v) was used as irritating agent. The negative control of the inhibition of irritation reaction was a saline isotonic solution (NaCl 0.9% w/v); the positive control for irritation inhibition reaction was a non-steroid anti-inflammatory drug, Nimesulida (AINEX®) at a concentration of 1 mg/mL. The anti-irritant property of the methanolic solution was also evaluated (solvent control). All of the solutions used in the HET-CAM test were daily prepared.
Standard irritation reaction
The procedure described by Luepcke, 1985 (16), was used, with modifications. Eggs were placed in a Nuaire AireGard 201/301 horizontal laminar flow cabinet. Egg shells were cut around the air sacs with a 3200 rpm circular saw with maximum blade capacity of 1/8''. Cut sections were removed with a forceps and dissecting tongs. The exposed CAM was humidified with 300 μL of isotonic NaCl solution and placed in an incubator for two hours, after which 200 μL of SDS 0.5% was placed on the CAM. Three irritation reactions (hemorrhage, lysis, and coagulation) were monitored and registered with a Sony DCR-DVD 610 video camera placed at 35 cm above the CAM. Time was recorded in seconds, from the addition of SDS (time = 0) until the appearance of the three irritation reactions.
Determination of the anti-irritant capacity
CAM of eggs was exposed as described above and pre-treated with 300 μL of testing samples or control solutions. To prevent contamination of the CAM surface, egg-shell openings were covered with paraffin film. Eggs were placed in the incubator for two hours to facilitate absorption by the membranes, and the CAM was then treated with 200 μL of SDS 0.5%. Time (in seconds) was monitored until appearance of the irritation reactions. The irritation index (IR) was calculated using the Equation 1.
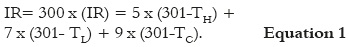
TH, TL and TC are, respectively, the time in seconds from the application of the irritant to the appearance of signs of hemorrhage (H), lysis (L), and coagulation (C) during a period of observation of 300 seconds. Because of the nature of the formula, IR can take values between 0 and 21. To determine the irritation potential of the substances, a classification analogous to the Draizé test was used: not irritating (IR= 0.0 – 0.9), slightly irritating (IR= 1.0- 4.9), moderately irritating (IR= 5.0 – 8.9), and severely irritating (IR = 9.0 – 21). The anti-irritating capacity H', L', and C' of the tested substances was expressed as the relationship between the starting times of irritation reactions in the CAM pre-treated with testing samples and negative control, using the Equations 2, 3 and 4 (12).

Statistical analysis
The HET- CAM test was triplicated for each test sample and controls, the data are expressed as mean ± SD. Two group comparisons were performed using Student's t test and statistical significance was accepted at the 95% confidence level (p < 0.025). All statistical evaluations were performed using IBM SPSS Statistics 19 for Windows (SPSS Inc., an IBM Company, Chicago, IL, USA).
RESULTS
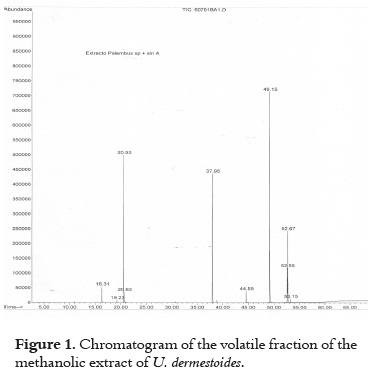
Figure 1 presents the chromatographic profile of the whole body methanolic extract of Ulomoides dermestoides.
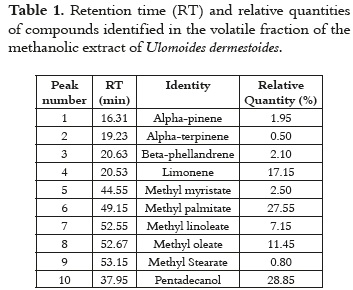
The molecules with the highest relative quantity were pentadecanol (28.85%), methyl palmitate (27.55%), limonene (17.15%), and methyl oleate (11.45%) which can be seen in Table 1.
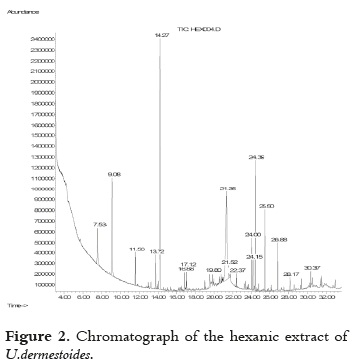
The chromatographic profile of the hexanic extract is shown in Figure 2.
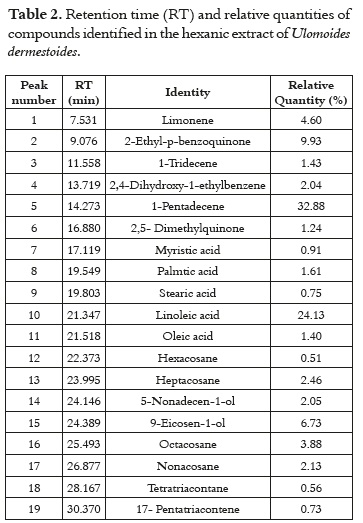
The compounds that were identified by GC-MS are shows in the Table 2. The compounds with the highest relative quantity were 1-pentadecene (32.88%), linoleic acid (24.13%), and 2-ethyl-p-benzoquinone (9.93%). Other compounds were 2,5-dimethylquinone, 2,4-Dihydroxy-1-ethylbenzene, limonene, aliphatic hydrocarbons, saturated and unsaturated fatty acids, and long-chain alcohols.
The changes of standard irritation reaction that occur in the CAM are shown in Figure 3. Hemorrhage reaction was observed in the aggrandizement of the major blood vessels and the appearance of small vascular complexes with multiple branches; lysis reaction was evidenced in the rupture of blood vessels and extravasations; clotting reaction was determined by the darkening and hardening of the CAM.
The inhibition of irritant reactions in the group pre-treat with positive control, methanolic extract of U. dermestoides and hexanic extract of U. dermestoides are shown in Figure 4.
The mean times for the beginning of the irritation reactions in CAM for each one of the tested samples and controls, as well as the mean values of the irritation index and anti-irritant capacity are shows in the Table 3.
DISCUSSION
The compounds 1- pentadecene, 2-ethyl-pbenzoquinone, limonene, and 1-tridecene identified in the extracts of U. dermestoides have been previously reported in studies to determine the chemical characteristics of the defensive secretions and sex-specific pheromones of U. dermestoides (17, 18). The monoterpene hydrocarbons alpha-pinene, alpha-terpinene, phellandrene, and dimethylquinones have been reported in the defensive secretions of some Australian tenebrionid beetles (19, 20). Insect defensive secretion is a mixture of repellent and blocking chemoreceptor substances that are stored in cuticular inclusions or abdominal glands, which are expelled when beetles are stressed. Defensive secretion of beetles has been postulated as a source of pharmacologically active compounds in treatment of respiratory diseases (8, 10, 21). Other compounds identified in the present study were saturated and unsaturated fatty acids and hydrocarbons with chains of 16-35 carbon atoms, some of these compounds have been found in the cuticle of beetles U. dermestoides and Blaps femoralis (17, 22) and they have been associated with important roles in the biology of beetles, such as sexual communication, preventing desiccation and protecting against pathogens.
In the HET-CAM test, pre-treatment of the CAM with methanolic extracts of U. dermestoides decreased the vascular hemorrhaging and membrane coagulation produced for the irritating agent (SDS 0.5%) (Mean of IR= 3.09 ± 0.11). There was a significant statistical difference in the irritation index between the group pre-treated with methanolic extract of U. dermestoides and negative control group, including the solvent control (p= 0.00). In addition, not significant statistical differences were seen in the hemorrhage reaction (p= 0.041), coagulation reaction (p= 0.028) and lyses reaction (p= 0.05) of the CAM between methanolic extract group and positive control group. Hexanic extract of U. dermestoides did not show any anti-irritant effect in the CAM test (Mean of IR= 20.6± 0.14). No statistical differences were observed in the index reaction between the hexanic extract group and negative control group (p = 0.00).
The potential anti-irritant capacity of methanolic extract of U. dermestoides can be attributed to the components with anti-inflammatory activity. Omega-9 monounsaturated fatty acid (MUFA) and omega-6 polyunsaturated fatty acid (PUFA) may contribute to the anti-irritant capacity of methanolic extracts. A systematic review of electronic databases and bibliographies of selected articles showed that diets rich in oleic acid (omega-9 MUFA) have beneficial effects on inflammation-related diseases (23). Bartoli et al., 2000 (24), reported that oleic acid can influence the metabolism of araquidonic acid (omega-6 PUFA), decreasing the production of pro-inflammatory eicosanoids. Moreover, the anti-inflammatory effect of linoleic acid (omega-6 PUFA) is not clear, several studies have expressed that a high intake of dietary linoleic acid contributes to excess of chronic inflammation. Linoleic acid can be metabolized to araquidonic acid, the substrate for the biosynthesis of a wide array of proinflammatory, vasoconstrictive, and/or proaggregatory eicosanoids; but the araquidonic acid is also the substrate for the production of anti-inflammatory and/or anti-aggregatory eicosanoids, such as prostacyclin, lipoxin A4 and epoxyeicosatrienoic acids (25). In the present study, the methanolic extract of U. dermestoides showed a higher quantity of oleic acid (11.45%) and lower quantity of linoleic acid (7.15%) compared with hexanic extract (1.4% and 24.13%, respectively). None anti-inflammatory or antiirritant activity has been reported for pentadecanol and methyl palmitate.
In addition, the methanolic extract has high quantity of monoterpenes (21.7%) with anti-inflammatory properties as limonene, alpha-terpinene and alpha-pinene (26). Recent findings suggest that Dlimonene could be used as a potential anti-inflammatory agent for the treatment of bronchial asthma by suppressing pro-inflammatory cytokines, radical oxygen species (ROS) production, and inactivating eosinophil migration; experiments with RAW 264.7 macrophage cells demonstrated that D-limonene is an effective inhibitor of lipopolysaccharide (LPS)- induced nitric oxide (NO) and prostaglandin E(2) production; likewise, D-limonene decreased the expression of pro-inflammatory cytokines TNFalpha, IL-1beta, and IL-6 in a dose-dependent manner (27). In addition, D-limonene obtained from a fruit peel of a traditional Japanese medicine named Yuzu (Citrus junos Tanaka) showed inhibition of the ROS production for eotaxin-stimulated HL-60 clones 15 cell at a low concentration; while at a higher concentration suppressed the cell chemotaxis and production of the monocyte chemotactic protein-1 (MCP-1) via NF-κB activation (28). The anti-inflammatory properties of alpha-pinene and alpha-terpinene have been demonstrated using an inflammatory experimental model in mice and an inhibition test of ovine cyclooxygenases (COX-1 y COX-2), respectively (29, 30).
These results about the anti-irritant capacity of methanolic and hexanic extracts of U. dermestoides are preliminary, because it is necessary to evaluate the effect of the extracts concentration on the CAM and the anti-irritant effect of the major components of each extract. In this study the hexanic extract used in the HET CAM test was a concentrated sample and this might influence the results. We propose that the high irritation index of the CAM pre-treated with hexanic extract could be caused by the aliphatic hydrocarbons and quinones (2-ethyl-p-benzoquinone and 2,5-dimethylquinone). Aliphatic hydrocarbons (43.85% of hexanic extract) may cause irritation of the skin and the mucous membranes (31), while quinones (11.17% of hexanic extract) have been reported as highly cytotoxic and/or genotoxic due to the formation of ROS and covalent binding to macromolecules (32, 33). However, the irritant effect of quinones present in the U. dermestoides extracts should be confirmed in further studies.
CONCLUSIONS
The whole-body methanolic and hexanic extracts of Ulomoides dermestoides presented volatile and semivolatile compounds that are characteristic of the defensive secretions and the cuticular surface of tenebrionids. The main compounds in the methanolic extract were fatty acids, pentadecanol and terpens. The methanoic extract exhibited anti-irritant capacity in a modified HET-CAM test that could be attributed to oleic acid and monoterpens like limonene; other minor compounds with anti-inflammatory activity were alpha-pinene and alpha-terpinene.
ACKNOWLEDGEMENTS
We are grateful to the Entomology Department of the Natural Sciences Institute (Universidad Nacional, Bogotá, Colombia) for scientific advice and to ACONDESA for technical support during this study. This investigation was conducted with the support of Universidad del Atlántico, Grupo de Productos Naturales y Bioquímica de Macromoléculas.
REFERENCES
1. Dacanay AA, Cervancia CR. Biology of Palembus (Martianus) dermestoides Chevrolat (Coleoptera; Tenebrionidae). Philippines Ent. 1989 Apr; 7 (5): 471-477. [ Links ]
2. Sandroni P. Aphrodisiacs past and present: a historical review. Clin Auton Res. 2001 Oct; 11 (5): 303-307. [ Links ]
3. Chu GS, Palmieri JR, Sullivan JT. Beetle-eating: a Malaysia folk medical practice and its public health implications. Trop Geogr Med. 1977 Dec; 29 (4): 422 - 427. [ Links ]
4. Costa Neto EM, Ramos-Elorduy J. Los insectos comestibles de Brasil: etnicidad, diversidad e importancia en la alimentación. Bol Soc Ent Aragonesa. 2006; 38: 423-442. [ Links ]
5. Costa-Neto EM. The use of insects in folk medicine in the state of Bahia Northeastern Brazil, with notes on insects reported elsewhere in Brazilian folk medicine. Human Ecol. 2002 Jun; 30 (2): 245-263. [ Links ]
6. Flores GE, Padín SB, Stetson RE. First records of the Oriental species Ulomoides dermestoides (Coleoptera: Tenebrionidae) in Argentina. Rev Soc Entomol Argent. 2002; 61 (3/4): 48-50. [ Links ]
7. Capul-Magaña FG. Sobre el uso de Ulomoides dermestoides (Chevrolat, 1878), (Coleoptera, Tenebrionidae, Diaperini) en la coleopteroterapia: informe de un caso en Ixtapa, Jalisco, México. Boln Asoc Esp Ent. 2010; 34 (3-4): 419-422. [ Links ]
8. Crespo R, Villaverde ML, Girotti JR, Güerci A, Juárez MP, de Bravo MG. Cytotoxic and genotoxic effects of defence secretion of Ulomoides dermestoides on A549 cells. J Ethnopharmacol. 2011 Jun 14; 136 (1): 204-209. [ Links ]
9. Santos RC, Lunardelli A, Caberlon E, Bastos CM, Nunes FB, Pires MG, et al. Anti-inflammatory and immunomodulatory effects of Ulomoides dermestoides on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation. 2010 Jun; 33 (3): 173-179. [ Links ]
10. Wahrendorf MS, Wink M. Pharmacologically active natural products in the defence secretion of Palembus ocularis (Tenebrionidae, Coleoptera). J Ethnopharmacol. 2006 Jun; 106 (1): 51-56. [ Links ]
11. Nia R, Paper DH, Essien EE, Oladimeji OH, Iyadi KC, Franz G. Investigation into In vitro radical scavenging and in vivo antiinflammatory potential of Tridax procumbes. Niger J Physiol Sci. 2003; 18 (1-2): 39-43. [ Links ]
12. Wilson TD, Steck WF. A modified HET-Cam assay approach to the assessment of anti-irritant properties of plant extracts. Food Chem Toxicol. 2000 Oct; 38 (10): 867-872. [ Links ]
13. Moura do Carmo DF, Amaral AC, Machado GM, Leon LL, Silva JR. Chemical and Biological Analyses of the Essential Oils and Main Constituents of Piper Species. Molecules 2012 Feb; 17: 1819-1829. [ Links ]
14. Unruh L, Rongda X, Kramer K. Benzoquinone levels as a function of age and gender of the red flour beetle, Tribolium casteneum. Insect Biochem Mol Biol. 1998 Aug; 28 (12): 969-977. [ Links ]
15. Bagley DM, Waters D, Kong BM. Development of a 10-day chorioallantoic membrane vascular assay as an alternative to the Draize rabbit eye irritation test. Food Chem Toxicol. 1994 Dec; 32 (12): 1115-1160. [ Links ]
16. Luepcke N.P. HET-chorioallantois-test: an alternative to the Draizé rabbit eye test. In: Goldberg, A.M. (Ed.), In Vitro Toxicology. New York, USA: Mary Ann Libert Inc. Publishers; 1985. 353-363 p. [ Links ]
17. Villaverde ML, Girotti JR, Mijailovsky SJ, Pedrini N, Juárez MP. Volatile secretions and epicuticular hydrocarbons of the beetle Ulomoides dermestoides. Comp Biochem Physiol B Biochem Mol Biol. 2009 Dec; 154 (4): 381-386. [ Links ]
18. Martins CBC, Zarbin PHG, Almeida LM. Evidence for sexspecific pheromones in Ulomoides dermestoides (Coleoptera, Tenebrionidar). Fla Entomol. 2010 Dec; 93 (4): 639-641. [ Links ]
19. Geiselhardt S, Schmitt T, Peschke K. Chemical composition and pheromonal function of the defensive secretions in the subtribe Stizopina (Coleoptera, Tenebrionidae, Opatrini). Chemoecology. 2009 Jun; 19 (1): 1-6. [ Links ]
20. Brown WV, Doyen JT, Moore BP, Lawren JF. Chemical composition and taxonomic significance of defensive secretions of some Australian Tenebrionidae (Coleoptera). J Aust Ent Soc. 1992 Feb; 31 (1): 79-89. [ Links ]
21. Howard RW, Jurenka RA, Blomquist GJ. Prostaglandin synthetase inhibitors in the defensive secretion of the red flour beetle Tribolium castaneum (Herbst) (Coleoptera:Tenebrionidae). Insect Biochem. 1986 Jun; 16 (5): 757-760. [ Links ]
22. Gunbilig D, Boland W. Defensive Agents of Blaps femoralis a Traditional Mongolian Medicinal Insect. Sci Pharm. 2009 Jul; 77 (3): 597-604. [ Links ]
23. Carrillo C, Cavia Mdel M, Alonso-Torre S. Role of oleic acid in immune system; mechanism of action; a review. Nutr Hosp. 2012 Jul; 27 (4): 978-990. [ Links ]
24. Bartoli R, Fernandez-Banares F, Navarro E, Castella E, Mane J, Alvarez M, et al. Effect of olive oil on early and late events of colon carcinogenesis in rats: modulation of arachidonic acid metabolism and local prostaglandin E2 synthesis. Gut. 2000 Feb; 46 (2): 191-199. [ Links ]
25. Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009 Feb; 119 (6): 902-907. [ Links ]
26. da Silveira R, Andrade L, de Sousa D. A Review on Anti- Inflammatory Activity of Monoterpenes. Molecules. 2013 Jan; 18 (1): 1227-1254. [ Links ]
27. Yoon WJ, Lee NH, Hyun CG. Limonene suppresses lipopolysaccharide- induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J Oleo Sci. 2010; 59 (8): 415-421. [ Links ]
28. Hirota R, Roger NN, Nakamura H, Song HS, Sawamura M, Suganuma N. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J Food Sci. 2010 Apr; 75 (3): H87-92. [ Links ]
29. Quintão NL, da Silva GF, Antonialli CS, Rocha LW, Cechinel Filho V, Cicció JF. Chemical composition and evaluation of the anti-hypernociceptive effect of the essential oil extracted from the leaves of Ugni myricoides on inflammatory and neuropathic models of pain in mice. Planta Med. 2010 Sep; 76 (13): 1411-1418. [ Links ]
30. Kawata, J.; Kameda, M.; Miyazawa, M. Cyclooxygenase-2 inhibitory effects of monoterpenoids with a p-methane skeleton. Int J Essent Oil Ther. 2008 Dic; 2 (4): 145-148. [ Links ]
31. Patlolla RR, Mallampati R, Fulzele SV, Babu RJ, Singh M. Dermal microdialysis of inflammatory markers induced by aliphatic hydrocarbons in rats. Toxicol Lett. 2009 Mar; 185 (3): 168-174. [ Links ]
32. Yang F, Zhou J. Cytotoxicity and DNA damage induced by 1, 4-benzoquinone in v79 Chinese hamster lung cells. J Toxicol Environ Health, Part A. 2010 Jan: 73 (7): 483-489. [ Links ]
33. Ruiz-Ramos R, Cebrian ME, Garrido E. Benzoquinone activates the ERK/MAPK signaling pathway via ROS production in HL- 60 cells. Toxicology. 2005 May; 209 (3): 279-287. [ Links ]