Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.20 no.2 Medellín May/Aug. 2013
FOODS: SCIENCE, TECHNOLOGY AND ENGINEERING
THE USE OF NATURAL ANTIOXIDANTS (OREGANO AND SAGE) TO REDUCE HEXANAL PRODUCTION IN PRECOOKED CHICKEN DURING CHILL STORAGE
USO DE ANTIOXIDANTES NATURALES (ORÉGANO Y SALVIA) PARA REDUCIR LA PRODUCCIÓN DE HEXANAL EN MUESTRAS DE POLLO PRECOCINADO Y CONGELADO
Lilian MARQUES PINO PhD1; Carlos CAVALEIRO PhD2; Maria da CONCEIÇÃO CASTILHO PhD2; Marisa Aparecida BISMARA REGITANO D'ARCE PhD3; Elizabeth Aparecida DA SILVA TORRES PhD4; Fernando RAMOS PhD4*
1 Instituto Federal Educação, Ciência e Tecnologia de São Paulo - Campus São Roque Rodovia Prefeito Quintino de Lima, 2100 - Paisagem Colonial, São Roque/SP CEP 18136-540, Brasil.
2 CEF – Centro de Estudos Farmacêuticos – Pólo das Ciências da Saúde, Faculdade de Farmácia, Univeridade de Coimbra, Azinhaga de Santa Comba, 3000-548 Coimbra, Portugal.
3 Escola Superior de Agricultura ''Luiz de Queiroz'' – USP Avenida Pádua Dias, 11- Piracicaba/SP CEP: 13418-900, Brasil.
4 Faculdade de Saúde Pública – USP Avenida Dr. Arnaldo, 715 - São Paulo/SP CEP:01246-904, Brasil.
* Autor a quien se debe dirigir la correspondencia: fjramos@ci.uc.pt.
Recibido: Abril 03 de 2013
Aceptado: Octubre 08 de 2013
ABSTRACT
Background: The properties of plants with food preservation potential are well known since the antiquity. In recent years, the use of herbs and spices to improve the sensory characteristics and to extend the shelf-life of foods has been growing. Objectives: To compare oregano (Origanum vulgare L.) and sage (Salvia officinalis L.) as a natural antioxidant in balls of chicken breast and added 0.50% salt. Methods: Samples of chicken meatballs were pre-cooked for 8 minutes in a water bath at 80° C, and packaged in polyethylene bags with three layers, specific for vacuum cooking and high temperatures. The samples were separated into three groups: control (just salt), 0.10% oregano (dry plant) and sage 0.10% (dry plant), stored at -20° C for 144 hours. The tests to verify the formation of hexanal in samples were performed in the period of 0, 48, 96 and 144 hours of refrigerated storage. The determination of hexanal, extracted by micro-extraction (headspace solid phase micro-extraction - HS-SPME), was used as an indicator of the lipid oxidation of the samples. The analysis was conducted by a gas chromatograph coupled with a mass detector (GC-MS). The method was evaluated according to the validation parameters such as linearity, repeatability and detection limit. The extraction was conducted at 70° C using a fiber (DVD / CAR / PDMS) exposed for 20 minutes in the headspace after 5 minutes of equilibrium between the sample and the headspace. Results: Samples added 0.10% oregano or sage had effective protection in the development of hexanal, compared to control samples. The development of hexanal was significantly higher in the control samples, 34 μg of hexanal/100 g of sample at the beginning and 280 µg/100 g sample at the end of refrigerated storage. Conclusion: Herbs were effective in controlling the development of hexanal. However, the oregano was more effective than sage in preventing the formation of hexanal in chicken meatballs pre-cooked. The level of hexanal in herbs at the end of the storage period was 5 μg hexanal/100 g sample for oregano and 23 µg hexanal/100 g sample for sage.
Keywords: Natural antioxidants, chicken meat, hexanal, oregano, sage.
RESUMEN
Antecedentes: Las propiedades de las plantas con potencial de conservación de los alimentos se conocen desde la antigüedad. El uso de hierbas y especias para mejorar las características sensoriales y prolongar la vida de los alimentos ha tenido un incremento considerable en años recientes. Objetivos: Comparar las propiedades antioxidantes del orégano (Origanum vulgare L.) y de la salvia (Salvia officinalis L.) en carne de pollo precocinada con 0,50% de sal. Métodos: Las muestras fueron separadas en tres grupos: control (sólo sal), 0,10% de orégano (planta seca) y 0,10% salvia (planta seca), almacenadas a -20°C durante 144 horas. Las muestras de pollo fueron envasadas en bolsas de polietileno con tres capas, específicas para la cocción al vacío y a altas temperaturas. Las muestras fueron pre-cocidas durante 8 minutos a 80°C. Se utilizó el hexanal como indicador de la oxidación lipídica. Los análisis para evaluar la formación de hexanal se realizaron en los tiempos de 0, 48, 96 y 144 horas de almacenamiento refrigerado. El hexanal se determinó por cromatografía de gases con detector de espectrometría de masas (GC-MS), después de una micro-extracción en fase sólida en espacio de cabeza (HS-SPME) a 70°C, utilizando una fibra (DVD/CAR/PDMS) expuesta durante 20 minutos, y después de 5 minutos de equilibrio entre la muestra y el espacio de cabeza. El método ha sido validado de acuerdo a los parámetros más comunes, tales como límite de detección, linealidad, y repetibilidad. Resultados: Ambas plantas secas (orégano y salvia) son eficaces en la prevención de la oxidación lipídica de la carne de pollo durante 114 horas. El desarrollo de hexanal fue significativamente mayor en las muestras control, 34 μg/100g al inicio y 280 μg/100g de muestra al final del almacenamiento refrigerado. Conclusiones: La adición de 0,10% de orégano o salvia a las muestras en estudio resultó eficaz en la protección contra el desarrollo de hexanal. Además, el orégano demostró, en el largo plazo, ser más eficiente que la salvia. El nivel de hexanal para las 144 horas de almacenamiento fue de 5 μg/100g para el orégano y de 23 μg/100g para la salvia.
Palabras clave: Antioxidantes naturales, pollo, hexanal, orégano, salvia.
INTRODUCTION
Lipid oxidation is one of the primary sources of quality deterioration in foods, mainly in meat products. This process is a decisive factor in the reduction of the shelf life of food products because it is responsible for the production of undesirable odors, texture deterioration, decrease in nutritional value and formation of potentially toxic substances (1-4). Pre-cooked meat and meat products reheated after a short period of refrigerated storage develop a distinctive off-flavor referred to as ''warmed-over flavor'' (WOF) (5). These undesirable odors and this flavor represent serious impediments to consumption. WOF development is largely attributed to the autoxidation of phospholipids and polyunsaturated fatty acids. The susceptibility and rate of phospholipid oxidation is dependent on the level of fatty acids present, and on their degrees of unsaturation. Rhee et al., 1996 (6), have shown that lipid oxidation of muscle foods occurs in the following sequence: fish > poultry (chicken and turkey) > pork > beef > lamb. This order corresponds to polyunsaturated fatty acids composition, decreasing from higher to lower levels (7).
To minimize meat oxidative deterioration, the addition of antioxidants to such products is the most common procedure (8-11). One variation is the use of extracts from herbs and spices or dry plants to improve the sensory characteristics and extend the shelf life of foods, intended to avoid the suspected carcinogenic potential of synthetic antioxidants. In that sense, some herbs and spices such as sage and garlic have been studied for their anti-oxidative activity in chicken meat (12).
The ability to evaluate lipid oxidation condition is of great interest for both the food industry and consumers. Torres et al., 1989 (13), studying the lipid oxidation in salted and dried beef, have demonstrated that hexanal is a good indicator of lipid oxidation. Therefore, hexanal has become a known indicator, as a major fat oxidation product that increases during storage (14). In the past few years, hexanal has been evaluated in several food matrixes, including cooked turkey (14), freeze dried chicken myofibrils (2), beef (15, 16), fish (17), vegetable oils (18), crisps (19) and cooked chicken (10, 20) as an indicator of lipid deterioration.
Headspace solid phase micro-extraction (HSSPME) has been employed to study the antioxidant properties of herb extracts with subsequent injection into a gas chromatographic mass detection (GC-MS) system. This approach was chosen because it is a well-known non-invasive and solventfree method, presenting major advantages, like simplicity, rapidity and high sensitivity using a small sample volume (15, 21, 22).
The aim of this study was to compare the properties of dry sage (Salvia officinalis L.) with those of dry oregano (Origanum vulgare L.), also identified as a natural antioxidant (7), in precooked meat balls made from chicken breast with 0.50% added salt during chill storage for up to six days, packed in atmospheric air.
MATERIALS AND METHODS
Reagents and Equipment
Hexanal and 1,4-dioxane (internal standard) were supplied from Sigma-Aldrich (Madrid, Spain) and had a purity above 99%. For SPME, a standard hexanal solution of 100 mg L-1 was prepared in ultrapure water, obtained with a Milli Q System (Millipore, Bedford, MA, USA).
For storage and sample preparation it was used an Electrolux freezer (Coimbra, Portugal), a Mettler AG285 balance (Zurich, Switzerland), micropipettes from Gilson (Villiers-le-Bell, France), a Moulinex mixer (Lisbon, Portugal) and a vortex type agitator (UnimagZX, Reagente 5, Oporto, Portugal).
Meat sample preparation
The meat samples are chicken breast balls purchased from a local supermarket. The meat was homogenized, and the balls consisted of 30 g of meat and 0.50% of salt, according to the method described by Racanicci et al., 2004 (23). The treatments were oregano (0.10%), sage (0.10%) and control (just salt). The preparation included a precooking of the balls for eight minutes at 80°C and a packaging. All samples were maintained in chill storage at –20 ± 2°C for six days and analyzed at 48, 96 and 144 hours of storage.
Determination of hexanal by HS-SPME-GC-MS
For the determination of hexanal, it was necessary to homogenize the chicken balls using a mixer. 2 g of sample was mixed with 1 mL of ultrapure water and 1 mL of 1,4-dioxane (0.2080 μg per 1mL) in a 20 mL vial. The vial was hermetically capped with a PTFE-faced silicone septum and vortexed for 3 min. After homogenization, samples were heated in an oven at 70°C for 5 minutes to establish the equilibrium between the sample matrix and the headspace. A fused silica-coated fiber (DVD-CARPDMS, Supelco, Bellefonte, PA, USA) assembled in an SPME holder (Supelco) was used to extract hexanal from the chicken meat. The fiber was exposed in the headspace for 20 minutes (extraction time) at 70°C and then immediately inserted into the GC injection port for thermal desorption at 250°C for 3 minutes.
GC-MS analyses were performed in an Agilent gas chromatograph 6890N coupled with to mass selective detector Agilent 5973 Network (Soquimica, Lisbon, Portugal). The injector, with a narrow-bore glass liner with 0.80 mm i.d., was operated at 250°C in splitless mode (1 min). An HP-Wax fused silica column (30 m x 0.32 mm i.d x 0.2 mm polyethyleneglycol film thickness) was used with helium (Sogafer, Coimbra Portugal) as the carrier gas, at a flow of 1ml min-1, for the chromatographic separations. Oven temperature was programmed from 50°C to 220°C at a rate of 3°C min-1. Mass spectra were acquired under the following MS parameters: interface temperature (250°C), source temperature (230°C), quadrupole temperature (150°C), ionization energy (70 eV), ionization current (60mA), scan range (35–350 units) and 4.51 scans/second.
Confirmation of hexanal was performed by comparing retention time and acquired mass spectra with that recorded in Wiley Spectrometry Library.
The results were expressed as µg hexanal/100 g of sample.
RESULTS
Method validation
For method validation, linearity, precision, accuracy and detection limit it was used blank meat samples spiked with 0.0, 0.5, 1.0, 2.0 and 5.0 μg mL-1 of hexanal and submitted to the sample preparation procedure described above. For each calibration level, three samples were prepared and analyzed daily for 3 days. Additionally, one standard calibration curve without matrix at the same calibration levels was prepared and analyzed each day.
The linearity (24, 25) was evaluated by the regression coefficients (r2) for the calibration curves and was always > 0.9905.
The precision in terms of repeatability and intermediate precision was evaluated by calculating the coefficient of variation (CV %) according to the ICH recommendations (25). In the evaluation of the intermediate precision, three concentration levels at 0.5, 1.0 and 2.0 μg mL-1 were used during the 3 days. The obtained coefficients of variation 3.60 (n = 3) and 6.13 (n = 9) for repeatability and intermediate precision, respectively, were considered acceptable (26). Reproducibility, according to the International Conference on Harmonization concept, was not evaluated because the method has yet to be applied in different laboratories (24, 25).
The accuracy was evaluated through recovery testing. Three chicken meat ball samples were spiked, in triplicate, with 0.5, 1.0 and 2.0 μg mL-1 of hexanal. The obtained mean recovery percentage was 99.60% (n = 9), which indicates that the method is accurate according to the acceptability criteria for this kind of determination (27).
The detection limit was estimated to be 1.00 ng mL-1 of hexanal, in accordance with ACS guidelines (28), and was similar to the data on the GC-MS method reported by Sanches-Silva et al., 2004 (19), and by Giuffrida et al., 2005 (15), for the determination of hexanal in potato crisps and in beef bouillons.
Validation data for the proposed methodology for hexanal determination indicate that HS-SPME/ GC-MS is a good procedure for determining and monitoring the development of this volatile compound as verified by other authors (15, 19, 21, 22).
Evolution of hexanal content during storage of chicken meat balls
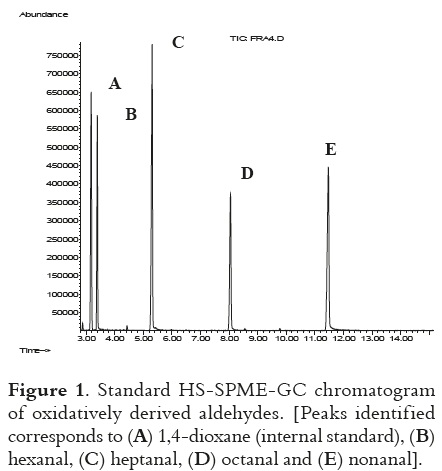
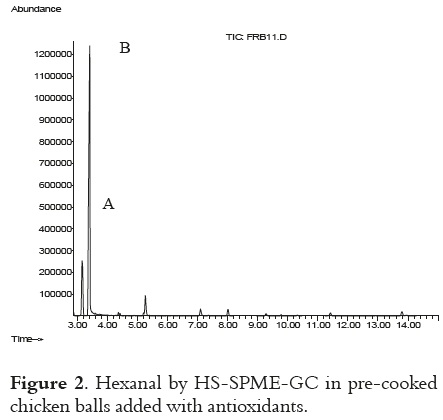
In Figure 1, a typical standard chromatogram of internal standard (1,4-dioxane), hexanal, heptanal, octanal and nonanal is shown. Byrne et al., 2002 (29), found these volatile compounds while studying the effect of oven cooking on warmedover flavors in chicken meat, but in this study it was clearly demonstrated that hexanal was the best indicator for lipid oxidation (figure 2).
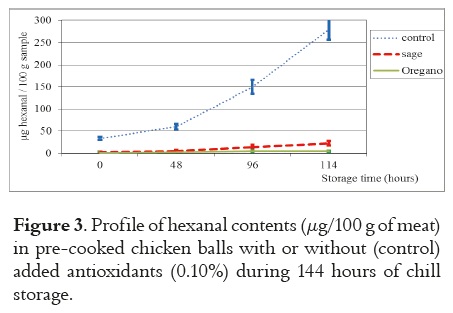
The evolution of the hexanal levels during the storage period are summarized in Figure 3, with the average values of three samples, each determined in triplicate along with error bars as standard deviations.
The data indicate that both the oregano and the sage additions were effective in decreasing hexanal levels through all periods of storage. The development of hexanal during the storage period is significantly higher in the control samples, as could be seen by the 34 mg of hexanal content per 100g of sample in the beginning (0 h) and 280 mg per 100g of sample at the end (114 h) of the storage period studied.
DISCUSSION
The HS-SPME is a non invasive procedure, solvent-free and has the advantages of simplicity, fast, sensitive, uses a small sample volume and is a good alternative to determine lipid oxidation. Several authors (16, 10) used to determine the hexanal as a measure to verify the oxidative stability of meat products, and report that the amount of hexanal in the samples showed a linear relationship with sensory attributes, such as the development of WOF (warmed-over-flavor).
An exponential increase in hexanal formation was observed after the second day of storage (48 h). High contents of lipid oxidation indicator are formed during the oxidation of linoleic acid via the 13-hydroperoxide. Also, a noticeable odor described as ''grassy'', that contributes to the warmed over flavor, was found (1). The higher susceptibility of chicken meat toward oxidation could be explained by its higher absolute content of polyunsaturated fatty acids, favoring hexanal development.
The hexanal levels in chicken meat with spices at the end of the storage period (144 h) were 5 mg and 23 mg per 100g of sample for oregano and for sage, respectively. The samples to which spices were added demonstrate a low development of hexanal, thus showing the great antioxidant effect of oregano and sage; the same relationship of these natural antioxidants was found by Sampaio et al., 2012 (10).
However, this antioxidant effect is not in agreement with results previously reported by Pizzale et al., 2002 (30), who stated that sage extracts had, on average, a higher antioxidant activity. However, conclusions made by Pizalle et al. were based on antioxidant activity evaluation of methanolic extracts of oregano and sage samples when submitted to rancimat and to crocin bleaching tests. Depending on the method used to test the antioxidant activity and on the specie tested, different results may be obtained. For crocin test, the two oregano species analyzed (O. indercedens and O. onites) had a higher antioxidant activity, whereas for the rancimat test, the two sage species assayed (S. officinalis and S. fruticosa) gave different results. Therefore, it's a bit difficult to establish which of these tests can better represent the oxidation of chicken meat balls.
Nevertheless, according to the data of the presented study, and confirmed by Fasseas et al., 2007 (31), oregano (Origanum vulgare L.) was revealed to be more efficient than sage (Salvia officinalis L.) in the prevention of long term hexanal development in pre-cooked chicken meat balls during chill storage for up to six days. Hence, the addition of oregano to pre-cooked chicken provides excellent anti-oxidative properties, as well as provides relevant data to be used for new product concepts by the food industry with potential gastronomic and economic profits.
CONCLUSION
Data of this study indicated that the addition of herbal dry were effective in controlling the development of hexanal, standing out the performance of oregano, during refrigerated storage of pre-cooked chicken breast samples.
ACKNOWLEDGMENTS
Authors would like to thank the State of São Paulo Research Foundation (FAPESP), the Brazilian National Council for Scientific and Technological Development (CNPq), the Center for Pharmaceuticals Studies (CEF) – Portugal, and the Postgraduate Program in Applied Human Nutrition (PRONUT) of the University of São Paulo for their support and for granting a scholarship to Lilian Marques Pino.
REFERENCES
1. Fenaille F, Visani P, Fumeaux R, Milo C, Guy P. Comparison of Mass Spectrometry-Based Electronic Nose and Solid Phase MicroExtraction Gas Chromatography MassSpectrometry Technique to Assess Infant Formula Oxidation. J Agr Food Chem. 2003; 51: 2790-2796. [ Links ]
2. Goodridge C, Beaudry R, Pestka J, Smith D. Solid Phase Microextraction- Gas Chromatography for Quantifying Headspace Hexanal Above Freeze-Dried Chicken Myofibrils. J Agr Food Chem. 2003; 51: 4185-4190. [ Links ]
3. Ripoll G, Joy M, Muñoz F. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Sci. 2011; 87: 88-93. [ Links ]
4. Trefan L, Bürger L, Bloom-Hansen J, Rooke JA, Salmi B, Larzul C, Terlouw C, Doeschl-Wilson A. Meta-analysis of the effects of dietary vitamin E supplementation on α-tocopherol concentration and lipid oxidation in pork. Meat Sci. 2011; 87: 305-314.
5. Bailey ME, Shin-Lee SY, Dupuy HP, St.Angelo AJ, Vercellotti JR. Inhibition of warmed-over flavor by Maillard reaction products. In Warmed-over Flavor of Meat, St.Angelo AJ & Bailey ME (eds.), Academic Press, Orlando, FL, USA, p. 237-266. [ Links ]
6. Rhee KS, Anderson, LM, Sams AR. Lipid oxidation potential of beef, chicken and pork. J Food Sci. 1996; 61: 8-12. [ Links ]
7. Botsoglu NA, Christaki E, Fletouris DJ, Florou-Paneri P, Spais AB. The effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002; 62: 259-265. [ Links ]
8. Trindade RA, Mancini-Filho J, Villavicencio ALCH. Natural antioxidants protecting irradiated beef burgers from lipid oxidation. LWT - Food Sci Technol. 2010; 43: 98-104. [ Links ]
9. Doolaege EHA, Vossen E, Raes K, De Meulenaer B, Verhé R, Paelinck H, De Smet S. Effect of Rosemary extract dose on lipid oxidation colour stability and antioxidant concentrations, in reduced nitrite liver pâtés. Meat Sci. 2012; 90: 925-931. [ Links ]
10. Sampaio GR, Saldanha T, Soares RAM, Torres, EAFS. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012; 135: 1383-1390. [ Links ]
11. Racanicci AMC, Menten JFM, Alencar, SM, Buissa RS, Skibsted, LH. Mate (Ilex paraguariensis) as dietary additive for broilers: performance and oxidative stability of meat. Eur Food Res Technol. 2011; 232: 655-661. [ Links ]
12. Mariutti LRB, Orlien V, Bragagnolo N, Skibsted LH. Effect of sage and garlic on lipid oxidation in high-pressure processed chicken meat. Eur Food Res Technol. 2008; 227: 337-344. [ Links ]
13. Torres EAFS, Pearson TAM, Gray JL, Ku PK, Shimokomaki M. Lipid oxidation in charqui (salted and dried beef). Food Chem. 1989; 32: 257-268. [ Links ]
14. Brunton NP, Cronin DA, Monahan FJ, Duncan R. A comparison of solid-phase microextraction (SPME) fibres for measurement of hexanal and pentanal in cooked turkey. Food Chem. 2000; 68: 339-345. [ Links ]
15. Giuffrida F, Golay P-A, Destaillats F, Hug B, Dionisi F. Accurate determination of hexanal in beef bouillons by headspace solidphase microextraction gas-chromatography mass-spectrometry. Eur Food Res Technol. 2005; 107: 792–798. [ Links ]
16. Olivares A, Dryahina K, Spanel P, Flores M. Rapid detection of lipid oxidation in beef muscle packed under modified atmosphere by measuring volatile organic compounds using SIFT-MS. Food Chem. 2012; 135: 1801-1808. [ Links ]
17. Veloso MCC, Silva VM, Santos GV, Andrade JB. Determination of Aldehydes in Fish by High-Performance Liquid Chromatography. J Chromatogr Sci. 2001; 39: 173-176. [ Links ]
18. Seppanen CM, Casallany AS. Simultaneous determination of lipophilic aldehydes by high-performance liquid chromatography in vegetable oil. J Am Oil Chem Soc. 2001; 78: 1253-1260. [ Links ]
19. Sanches-Silva A, Rodríguez-Bernaldo de Quirós A, López- Hernández J, Paseiro-Losada P. Determination of hexanal as indicator of the lipidic oxidation state in potato crisps using gas chromatography and high-performance liquid chromatography. J Chromatogr A. 2004; 1046: 75-81. [ Links ]
20. Beltran E, Pla R, Yuste J, Mor-Mur M. Use of antioxidants to minimize rancidity in pressurized and cooked chicken slurries. Meat Sci. 2004; 66: 719-725. [ Links ]
21. Czerwiński J, Bogdan Zygmunt J, Namieśnik J. Head-space solid phase microextraction for the GC-MS analysis of terpenoids in herb based formulations. Fresen J Anal Chem. 1996; 356: 80-83. [ Links ]
22. Purcaro G, Moret S, Conte LS. HS-SPME-GC applied to rancidity assessment in bakery foods. Eur Food Res Technol. 2008; 227: 1-6. [ Links ]
23. Racanicci AMC, Danielsen B, Menten JFM, Regitano-d'Arce MAB, Skibsted LH. Antioxidant effect of dittany (Origanum dictamnus) in pre-cooked chicken meat balls during chill-storage in comparison to rosemary (Rosmarinus officinalis). Eur Food Res Technol. 2004; 218: 521-524. [ Links ]
24. ICH, International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use. ICH Q2A: Validation of Analytical Procedures: Text and Methodology. Rockville, MD, USA, FDA, 1994, 13 p. [ Links ]
25. ICH, International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use. ICH Q2B: Validation of Analytical Procedures: Methodology. Rockville, MD, USA, FDA, 1996. 10 p. [ Links ]
26. Bressolle F, Bromet-Petit M, Audran M. Validation of liquid chromatographic and gas chromatographic methods. J Chromatogr B. 1996; 686: 3-10. [ Links ]
27. Alder, L., Holland, P.T., Lantos, J., Lee, M., MacNeil, J.D., O'Rangers, J., van Zoonen, P., & Ambrus, A. Guidelines for Single-Laboratory Validation of Analytical Methods for Tracelevel Concentrations of Organic Chemicals in Principles and Practices of Method Validation, Fajgelj, A., & Ambrus, A. (ed.). The Royal Society of Chemistry, Cambridge, UK, 2000, p. 179-248. [ Links ]
28. ACS, American Chemical Society, Subcommittee of Environmental Analytical Chemistry. Guidelines for Data Acquisition and Data Quality Evaluation in Environmental Chemistry. Anal Chem. 1980; 52: 2242-2249. [ Links ]
29. Byrne DV, Bredie WLP., Mottram DS, Martens M. Sensory and chemical investigations on the effect of oven cooking on warmed-over flavour development in chicken meat. Meat Sci. 2002; 61: 127-139. [ Links ]
30. Pizzale L, Bortolomeazzi R, Vichi S, Überegger E, Conte LS. Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O indercedens) extracts related to their phenolic compound content. J Sci Food Agr. 2002; 82: 1645–1651. [ Links ]
31. Fasseas MK, Mountzouris KC, Tarantilis PA, Polissiou M, Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2007; 106: 1188–1194. [ Links ]