Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Vitae
versão impressa ISSN 0121-4004
Vitae vol.21 no.1 Medellín jan./abr. 2014
FARMACOLOGÍA Y TOXICOLOGÍA
ISOLATION AND FUNCTIONAL CHARACTERIZATION OF A BASIC PHOSPHOLIPASE A2 FROM COLOMBIAN Bothrops asper VENOM
AISLAMIENTO Y CARACTERIZACIÓN FUNCIONAL DE UNA FOSFOLIPASA A2 BÁSICA DEL VENENO DE Bothrops asper DE COLOMBIA
Jaime Andrés Pereañez. PhD1*, Juan Carlos Quintana. PhD2, Juan Carlos Alarcón. PhD1, Vitelbina Núñez. PhD3
1 Programa de Ofidismo/Escorpionismo, Sede de Investigación Universitaria (SIU), Universidad de Antioquia, Laboratorio 631, Departamento de Farmacia, Facultad de Química Farmacéutica, Universidad de Antioquia UdeA, Calle 70 No. 52-21, Medellín, Colombia.
2 Facultad de Medicina. Universidad Cooperativa de Colombia. Medellín-Colombia.
3 Escuela de Microbiología. Universidad de Antioquia UdeA, Calle 70 No. 52-21, Medellín, Colombia.
* Autor a quien se debe dirigir la correspondencia: andrespj20@yahoo.es.
Recibido: Septiembre 20 de 2013
Aceptado: Enero 28 de 2014
ABSTRACT
Background: Snakebites represent a relevant public health issue in many regions of the world, particularly in tropical and subtropical countries of Africa, Asia, Latin America and Oceania. Snake venoms are complex mixtures of toxic enzymes and proteins, where the most important and abundant muscle-damaging components in snake venoms are phospholipases A2 (PLA2s). Objective: Isolate and characterize a phospholipase A2 from Colombian Bothrops asper venom, in order to obtain information about venom composition of this species. Materials and methods: Cation-exchange chromatography followed by reverse phase HPLC were used to purify the protein. Mass spectrometry was used to determine its molecular mass. Biochemical characterization was performed using a synthetic substrate (4-nitro-3-octanoyloxy-benzoic acid). Myotoxic and edema-inducing activity of toxin were tested in mice, by measuring the plasma creatine kinase activity and footpad diameter, respectively. Moreover, cytotoxic activity was examined to murine skeletal muscle C2C12 myoblasts and myotubes. Results: A PLA2 of Bothrops asper venom from Colombia (BaspCol-PLA2) was purified. Its molecular mass was 13974.6 Da. The enzyme hydrolyzed a synthetic substrate with a KM of 3.11 mM and a VMax of 4.47 nmol/min, showing maximum activity at 40 °C and at pH 8.0. The PLA2 required Ca2+ for activity. The addition of Mg2+, Cd2+, Mn2+ and Zn2+ (10mM) in the presence of low Ca2+ concentration (1mM) decreased the enzyme activity. The substitution of Ca2+ by mentioned divalent cations also reduced the activity to levels similar to those in the absence of Ca2+. Three internal fragments (CCFVHDCCYGK, AAAI/ LCFRDNI/LNTYNDKK, DAAI/LCFR) identified by a mass spectrometry analysis showed similarity with previously reported B. asper PLA2s. In mice, BaspCol-PLA2 induced a conspicuous local myotoxic effect and moderate footpad edema. In vitro, this enzyme induced cytotoxic effect on both myoblasts and myotubes. Additionally, it was classified as weakly anticoagulant PLA2, showing this effect at concentrations between 3 and 10 μg/mL when using human plasma. Conclusions: A PLA2 was purified and named BaspCol-PLA2, this enzyme displayed catalytic activity and molecular mass of 13974.6 Da. The toxin showed myotoxic, edema-forming, anticoagulant and cytotoxic activities.
Keywords: Snake venoms, snake bites, Bothrops asper, phospholipases A2, necrosis, Colombia.
RESUMEN
Antecedentes: Los accidentes ofídicos representan un grave problema de salud pública en muchas regiones del mundo, particularmente en países tropicales y subtropicales de África, Asia, América latina y Oceanía. Los venenos de serpiente son mezclas complejas de enzymas y proteínas tóxicas, donde las fosfolipasas A2 (PLA2s) son uno de los principales y más abundantes componentes que destruyen el tejido muscular. Objetivo: Aislar y caracterizar una fosfolipasa A2 del veneno de Bothrops asper de Colombia, con el fin de obtener información acerca de la composición del veneno de esta especie. Materiales y Métodos: Se empleó cromatografía de intercambio catiónico seguida de HPLC en fase reversa para purificar la proteína. La masa molecular fue determinada por espectrometría de masas. La caracterización bioquímica fue llevada a cabo usando un sustrato sintético (ácido 4-nitro-3-octanoyloxi-benzoico). La actividad miotóxica y edematizante fue ensayada en ratones, al medir la actividad de la creatina kinasa en plasma y el aumento del diámetro de la almohadilla plantar, respectivamente. Además, la actividad citotóxica fue examinada en mioblastos y miotubos murinos C2C12. Resultados: Fue purificada una fosfolipasa A2 básica (BaspCol-PLA2) del veneno de Bothrops asper de Colombia. Su masa molecular fue 13974,6 Da. La enzima hidrolizó un sustrato sintético con un KM de 3,11 mM y un VMax de 4,47 nmol/ min, mostrando actividad máxima a 40 °C y pH 8,0. La PLA2 requirió Ca2+ para su actividad. La adición de Mg2+, Cd2+, Mn2+ y Zn2+ (10mM) en presencia de una baja concentración de Ca2+ (1mM) disminuyó la actividad enzimática. La sustitución de Ca2+ por otros cationes divalentes también redujo la actividad a niveles similares a aquellos presentados en ausencia de Ca2+. Tres péptidos internos (CCFVHDCCYGK, AAAI/LCFRDNI/LNTYNDKK, DAAI/LCFR) identificados por espectrometría de masas mostraron similitud con otras fosfolipasas A2 de B. asper previamente descritas. Cuando BaspCol-PLA2 fue inyectada en ratones indujo una miotoxicidad local considerable y un edema moderado. In vitro, esta enzima provocó efecto citotóxico sobre mioblastos y miotubos. Adicionalmente, esta proteína fue débilmente anticoagulante, mostrando este efecto sobre plasma humano en concentraciones entre 3 y 10 μg/mL. Conclusiones: Una PLA2 fue purificada y llamada BaspCol-PLA2, esta enzima presentó actividad catalítica y una masa molecular de 13974,6 Da. La toxina mostró actividad miotóxica, edematizante, anticoagulante y citotóxica.
Palabras clave: Venenos de serpiente, mordeduras de serpiente, Bothrops asper, fosfolipasas A2, necrosis, Colombia.
INTRODUCTION
Snakebites represent a relevant public health issue in many regions of the world, particularly in tropical and subtropical countries of Africa, Asia, Latin America and Oceania (1). Bothrops asper is responsible for 50–80% of snakebites, and 60-90% of deaths attributable to snakebites in Central America and Northern South America (2). Envenoming by this species induces marked local tissue damage that includes pain, edema, hemorrhage, and myonecrosis (2). The most important and abundant muscledamaging components in snake venoms are phospholipases A2 (PLA2; EC 3.1.1.4). These enzymes hydrolyze sn-2 ester bond of glycerophospholipids, releasing a fatty acid and a lysophospholipid (3). In addition, PLA2s can induce several pharmacological effects such as edema, modulation of platelet aggregation, as well as neurotoxicity and anticoagulation (3, 4). Snake venom PLA2s are classified into groups I or II, based on their sequence and mode of disulphide pairings. Group I PLA2s are found in the venoms of Elapidae snakes, whereas group II PLA2s are present in the venoms of Viperidae snakes (4). The group II is further divided into two main subgroups: Asp49 and Lys49 (PLA2 homologues) variants. In the latter, the aspartic acid residue at position 49, critically involved in calcium binding and essential for catalytic activity, is replaced by lysine. Due to this and other critical substitutions, the Lys49 PLA2s cannot bind calcium efficiently and are considered enzymatically inactive (5, 6). Although catalytic activity has shown to play a role in the toxic actions of some venom PLA2s, it is not essential in the case of Lys49 PLA2s, which use non-enzymatic mechanisms to alter membrane homeostasis (6).
Several PLA2s have been identified from B. asper venom including acidic and basic phospholipases A2. Ferlan and Gubensek (7) purified an acidic enzyme (PLA2 I) from the venom of Costa Rica's specimens. Alagón et al (8) characterized three acidic isoforms from the venom of B. asper from Mexico, named PLA2 1, PLA2 2 and PLA2 3. Recently, Fernández et al. (9) isolated and characterized an acidic enzyme (BaspPLA2-II) of B. asper from the Pacific region of Costa Rica. All of these isoforms are Asp49. The basic isoforms known as myotoxins I (Asp49) (10), II (Lys49) (11), III (Asp49) (12) and IV (Lys49) (13) have been isolated from the venom collected in Costa Rica, while another basic PLA2 isolated from this species of unspecified origin was reported by Mebs and Samejima (14). In addition, a cDNA coding for an Lys49 isoform of Costa Rican B. asper was cloned and its sequence deposited in GenBank (AAF14241; = UniProtKB Q9PVE3, unpublished). This diversity of PLA2 isoforms found in B. asper venom is in agreement with recent proteomic studies that evidenced marked geographical, ontogenetic, and individual venom variations (15).
On the other hand, the issue of intra-species venom variability has relevant implications for antivenom production, especially in species with wide geographic distribution as B. asper, which is distributed from southern Mexico to northern regions in South America (2). In the same way, despite that antivenoms could be effective in neutralize venoms from snake of geographically separated populations, it has been demonstrated that antivenoms tend to be more effective in the neutralization of homologous venoms (16). Additionally, differences in the immune response of horse to various types of venom components have been observed, especially to some P-I SVMPs and PLA2s (17). Thus, is important to know the composition of regional venom. In the present work, a basic PLA2 (BaspCol-PLA2) from the venom of Colombia's B. asper has been isolated and characterized, in order to obtain insights into its possible biological roles and its relevance to the pathophysiology of envenoming by this species in the Northwest region of the country. In addition, this work looks for new information about composition of B. asper venom from Colombia, the biochemical and toxicological profile of an isolated PLA2 and its comparison with other PLA2s isolated from B. asper venom and other snakes from Bothrops genus.
MATERIAL AND METHODS
Venom and animals
The venom was obtained by manual extraction of 18 specimens from Antioquia, northwest region of Colombia, maintained in captivity at the Serpentarium of the Universidad de Antioquia (Medellín, Colombia). Venoms were centrifuged at 3000 rpm for 10 min, and supernatants were lyophilized and stored at -20°C until used. For in vivo assays, Swiss Webster mice, 18–20 g body weight, were used. All experiments were conducted in accordance with guidelines of the Universidad de Antioquia Ethics Committee.
Isolation of PLA2
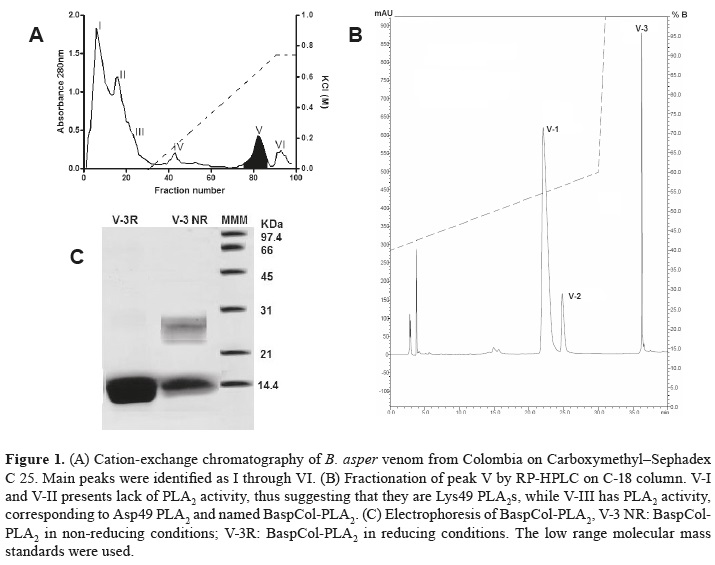
Two hundred and fifty mg of B. asper venom were diluted in 0.05 M Tris, 0.1 M KCl (pH 7.0) and applied to a Carboxymethyl - Sephadex C 25 column (1.8 cm x 30 cm), which had been preequilibrated with the same buffer. Proteins were eluted at a flow rate of 1.0 mL/min with a KCl gradient from 0.1 to 0.75 M (10), and elution profile was monitored at 280 nm.The fractions corresponding to main peaks were pooled, lyophilized, evaluated by PLA2 activity and sodium dodecylsulphatepolyacrylamide gel electrophoresis (SDS-PAGE). Then, five milligrams of basic fraction containing PLA2 activity were dissolved in 0.25 M ammonium bicarbonate at pH 8.0, and applied to a C-18 column (Shimadzu) for RP-HPLC. Proteins were eluted with a linear gradient from 0 to 66.0% (v/v) acetonitrile containing 0.1% (v/v) trifluoroacetic acid, at a flow rate of 1.0 ml/min. The elution profile was monitored at 280 nm in a UV/VIS photodiode array detector (Shimadzu) and fractions were manually collected, lyophilized and stored at -20°C.
Electrophoresis and molecular mass determination
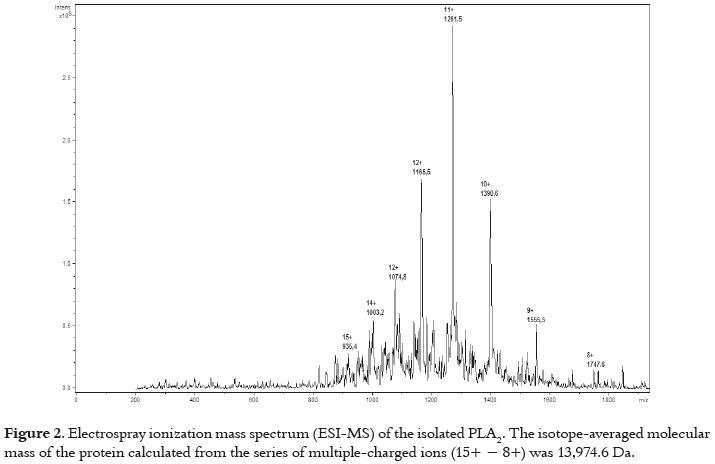
SDS-PAGE was performed on 15 % gels, under non-reducing or reducing (2-mercaptoethanol, 5%, v/v) conditions (18). Proteins were stained with Comassie blue R-250.The molecular mass of BaspCol- PLA2 was determined by electrospray ionization mass spectrometry (ESI-MS) on an Ion Trap LC/ MS 1200 series (Agilent Technology) operated in Enhanced Multiple Charge positive mode in the range m/z 200-4000.
PLA2 activity
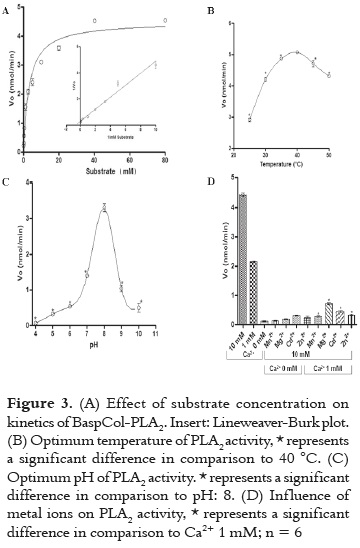
PLA2 activity was measured using the assay described by Cho and Kézdy (19) and Holzer and Mackessy (20), modified for 96-well plates. The standard assay mixture contained 200 μL of buffer (10 mMTris–HCl, 10 mM CaCl2, 100 mMNaCl, pH 8.0), 20 μL of substrate at different concentrations (4-nitro-3-octanoyloxy-benzoic acid), 20 μL of water and 20 μL of PLA2 (at 1 μg/ μL) in a final volume of 260 μL. After the addition of PLA2 (20 μg), the mixture was incubated 40 min at 37 °C, and the absorbance was read at 10 min intervals. The optimum pH and temperature of the PLA2 were determined by incubating the enzyme in buffers (10 mM citrate, 10 mM phosphate, 10 mMTris, and glycine 10 mM) of different pH (4.0-9.0), and in 10 mMTris–HCl, pH 8.0, at different temperatures (25-45 °C). The effect of substrate concentration on enzyme activity was determined by measuring the absorbance increase after 20 min of incubation in 10 mMTris–HCl, pH 8.0, at 37 °C. The enzyme activity, expressed as the initial velocity of the reaction (Vo), was calculated based on the increase in absorbance after 20 min. All assays were conducted in triplicate, and the absorbances read at 425 nm (Awareness, Stat Fax 3200).
Myotoxic activity
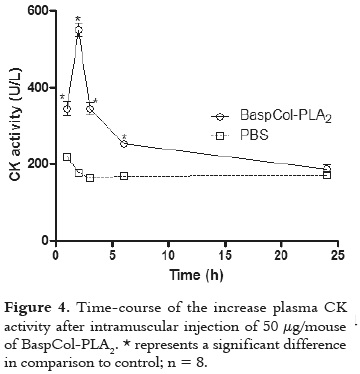
Groups of four mice received an intramuscular (i.m.) injection of 50 μg of toxin diluted in 100 μL of PBS (0.12 M NaCl, 0.04 M sodium phosphate, pH 7.2), in the gastrocnemius. A control group received 100 μL of PBS. At different time intervals (1, 2, 3, 6, 12, and 24 hr) the blood was collected from the tail into heparinized capillary tubes, and the plasma creatine kinase (CK; EC 2.7.3.2) activity was determined by a kinetic assay (Wiener Lab, CK-NAC UV-AA). Activity was expressed in U/L, one unit defined as the phosphorylation of 1 μmol of creatine/min at 25 °C.
Edema-forming activity
Groups of four mice received a subcutaneous (s.c.) injection of 10, 20, 40 and 80 μg of PLA2 in 50 μL of PBS, into the right footpad. The left footpad received 50 μL of PBS, as a control. After 2 h, footpad thickness was measured with a caliper, in millimeters. Edema was expressed as the increase percentage in thickness of the right foot, as compared to the left one, and the minimum edemaforming dose (MED) was defined as the toxin dose inducing 30% edema. Experiments were carried out in duplicate. The time-course analysis of edema was performed by injecting one MED in the right footpad of mice. The left footpad received 50 μL of PBS, as a control. Then, the edema was measured at 1, 2, 3, 6 and 24 h as described.
Cytotoxic activity
Cytotoxic activity was assayed on murine skeletal muscle C2C12 myoblasts and myotubes (ATCC CRL-1772) as described Lomonte et al. (21). Several amounts of toxin (5, 10, 20, 40 μg) were diluted in 150 μL of assay medium (Dulbecco's Modified Eagle's Medium supplemented with 1% fetal calf serum) and added to cells in 96-well plates. Controls for 0 and 100% toxicity consisted of assay medium and 0.1% Triton X-100, respectively. After 3 h at 37 °C, a supernatant aliquot was collected for determination of lactic dehydrogenase (LDH; EC 1.1.1.27) activity released from damaged cells, using a kinetic assay (Wiener LDH-P UV).
Anticoagulant activity
Different PLA2 amounts (0.15-10 μg) diluted in 100 μL of PBS (3.1-100 μg/mL) were added to 0.5 mL of human plasma, and incubated for 10 min at 37 °C. Plasma aliquots incubated with PBS were used as control. Then, coagulation times were recorded after adding 0.1 mL of 0.25 M CaCl2, in several assays (n= 5) (22). These doses were selected in order to determine whether the PLA2 is a strong, weak or non-anticoagulant enzyme, as defined by Kini (23).
Protein identification by HPLC-nESI-MS/MS.
Purified lyophilized protein was diluted in 8 M urea containing 10 mM DTT at pH 8.0, and the disulfide bridges were then reduced by incubation at 37 °C for 2 h. Iodoacetamide was used for alkylating the free thiols of cysteine residues, a 25% molar excess of iodoacetamide, relative to the total number of thiols, was eventually chosen and the mixture was incubated for 1.5 h at 37 °C in the darkness. The reaction was stopped by injecting the mixture onto a RP-HPLC column, followed by lyophilization of the collected peak. Afterwards, ten micrograms of isolated PLA2 was hydrolyzed with sequencing grade bovine pancreatic trypsin in 0.4% ammonium bicarbonate, pH 8.5, for 4 h at 37 °C, at an enzyme:substrate ratio of 1:100 (w/w). Then, the digestion product was subjected to nano HPLC column C-18 in a Mass Spectrometer LC/ MSD IonTrap 1200 series (Agilent Technology). The results of the mass spectra of peptides were run in the program Spectrum Mill (Agilent Technology) and Mascot (MatrixScience) in the NCBInr protein databases. Peptide sequences were searched for similarity using BLAST, and the sequences of PLA2s isolated from B. asper venom were obtained from Uni-Prot and aligned with identified peptides using the program ClustalW (24).
Statistical analysis
Significance of the differences recorded in enzymatic assays were analyzed by one-way ANOVA, followed by Bonferroni´s test. In cytotoxic and myotoxic activities, two-way ANOVA followed by Bonferroni´s test was applied. One-way ANOVA followed by Dunnet´s test was carried out in anticoagulant activity. In all cases, p < 0.05 was considered significant, and the results are shown as mean ± SEM of n indicated in each case. It was used the software SPSS 14.0 (http://www.spss.com; SPSS Inc. 233 South Wacker Drive, 11th Floor, Chicago, IL 60606-6412).
RESULTS
Isolation of PLA2
Figure 1A shows the Cation-exchange chromatography on CM-Sephadex C-25 of B. asper venom, which separated them into six fractions (I - VI). Myotoxic and PLA2 activities were restricted only to fraction V, which was separated by RP-HPLC into fractions V-1 through V-3, as shown in figure 1B. These fractions showed myotoxic activity when they were injected in mice. However, PLA2 activity was displayed only by fraction V-3. This suggests that V-1 and V-2 could be Lys49 PLA2s, while V-3 could be an Asp49 PLA2. Fraction V-3 was named BaspCol- PLA2. The molecular mass of PLA2 estimated by SDS-PAGE was ~ 14 KDa as displayed figure 1C. This was confirmed by ESI-MS, where molecular mass obtained was 13974.6 Da, as indicates figure 2.
Enzymatic characterization
The PLA2 activity of BaspCol-PLA2 was studied using the monodiperse substrate 4-nitro- 3-(octanoyloxy) benzoic acid. Under the conditions used, PLA2 showed a Michealian behavior indicated in figure 3A. The VMax was estimated to be 4.47 nmol/ min and the KM was 3.11 mM. figures 3B and 3C displayed that maximum enzyme activity occurred at 40 °C and the optimum pH was 8.0, respectively. In addition, in figure 3D is evidenced that BaspCol-PLA2 showed a strict dependence on calcium ions and it was active in concentrations of 1 and 10 mM Ca2+, with highest activity obtained with 10 mM (p <0.05). On the other hand, the addition of Mg2+, Cd2+, Mn2+ and Zn2+ (10 mM) in the presence of low Ca2+ concentration (1mM) decreased the enzyme activity. The substitution of Ca2+ by these other divalent cations also reduced the activity to levels similar to those in the absence of Ca2+.
Biological activities
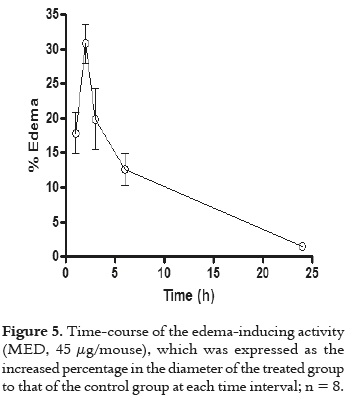
As displayed in figure 4, BaspCol-PLA2 induced a conspicuous myotoxic effect, evidenced by the rapid elevation of plasma CK activity, which reached a maximum 2 h after injection, and returned to normal by 24 h. In addition, in figure 5 is evidenced that this enzyme also induced moderate footpad edema, with a MED of 45.2 ± 2.4 μg, at 2 h, evidencing the local increase in vascular permeability. Time-course analysis of edema showed that this effect reached its highest point after 2 h and returned to normal by 24 h.
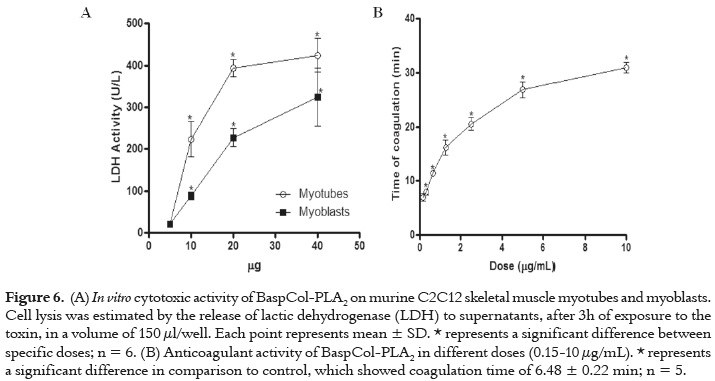
Figure 6A shows that the PLA2 induced cytotoxicity on myoblast as well as on myotubes, with a significantly higher effect recorded with the latter at doses of 10, 20 and 40 µg/well (p<0.05). As shown in figure 6B, BaspCol-PLA2 had anticoagulant effect on human platelet-poor plasma. Doses of 0.31 μg/100 μL (3.1 μg/mL) or higher caused a delay in clot formation (8-30 min), whereas 0.15μg/100 μL (1.5 μg/mL) of toxin did not delay coagulation time (p>0.05). Clot formation in control samples occurred at 6.48 ± 0.22 min.
Protein identification
After protein digestion and peptide separation in a nano C-18 HPLC column, some peptides were identified: CCFVHDCCYGK, AAAI/LCFRDNI/ LNTYNDKK, DAAI/LCFR by HPLC-nESI-MS/MS. The mono-isotopic masses of peptides were: 1505.5 Da, 2014.1 Da and 852.4 Da. Lysine residues shown in bold were deduced on the cleavage and missed cleavage by trypsin, and Cystein residues are shown as alkylated cysteine (C). These peptides matched with a number of viperid snake venom PLA2s as determined by a BLAST search. The active site of BaspCol- PLA2 was identified in the first peptide. Figure 7 display an alignment of the peptides identified in BaspCol-PLA2 with other PLA2s isolated from B. asper venom. The toxin from Colombian B. aper venom showed some identity (48-60%) with corresponding segments of other PLA2s isolated from Costa Rica's B. asper. 10-13)
DISCUSIÓN
A myotoxic PLA2, named BaspCol-PLA2, was purified from the venom of B. asper of the northwestern region from Colombia. The elution profile was comparable to other that used the similar conditions for isolating myotoxins from Bothrops venoms (10, 11, 25). This basic PLA2 showed enzymatic activity on monodisperse substrate (VMax: 4.47 nmol/min and KM: 3.11 mM), with a strict requirement of Ca2+, and maximum activity at pH 8.0 and 40 °C. These characteristics are common to other bothropic and crotalic PLA2s (26-28), and strongly suggest that position 49 of BaspCol-PLA2 corresponds to Asp49, as was demonstrated in one of the identified peptides (CCFVHDCCYGK). The KM value of BaspCol-PLA2 is higher in comparison with those calculated for other PLA2s by using non-monodispersed substrates (i.e. using aggregated substrate). This is explained by the preference of PLA2s for aggregated substrates, such as glycerophospholipids membrane. In addition, these enzymes suffer an interfacial activation when aggregated substrates (3-5). It is known that snake venom PLA2s are enzymes that resist extreme values of pH and temperature (3), which is in agreement with the values of pH and temperature of maximum activity of BaspCol-PLA2.
Injection of B. asper venom induces local myonecrosis in mice (29-31), in agreement with clinical observations (2). The most important and abundant muscle-damaging components in this venom are basic PLA2s and PLA2 homologues, as demonstrated by neutralization experiments (32). These toxins disrupt the integrity of muscle cell plasma membrane, and although details of this mechanism remain unknown at molecular level, it is clear that catalytically-dependent and independent events are involved (6, 33). Plasma CK activity reached a maximum at 2 h after injection of BaspCol-PLA2. Similar results were obtained with D49 PLA2s BbTx-III from B. brazili (26) and Cdcum6 from Colombian Crotalus durrissus cumanensis (27). However, slightly different results were obtained with other D49 PLA2s from Bothrops venoms, such as myotoxin I from Costa Rican B. asper venom, whose maximum myotoxic activity occurred three hours after its injection (10). Likewise, D49 PLA2s BmjeTx-I and II from B. marajoensis caused maximum of plasma CK activity six hours after their injection (28). Phospholipid hydrolysis by Asp49 PLA2s plays a critical role in muscle fiber plasma-membrane destabilization, since alkylation of His48 decreases myotoxic effect (34). The partial inhibition after catalytic inactivation suggests that molecular regions distinct from the catalytic site are able to interact and disrupt the integrity of muscle fiber plasma-membrane. The identity of these molecular regions remains largely unknown. Observations with myotoxic Asp49 PLA2s agree with the general hypothesis proposed by Kini and Evans (35) to explain the pharmacological profile of venom PLA2s, i.e. that these enzymes have in addition to the active site some molecular regions that determine their toxicity and tissue specificity. Small differences in these regions and in the catalytic properties could explain the differences mentioned above to induce myotoxic effect. In the same way, the use of cell cultures, such as rodent lines of skeletal muscle myoblasts/myotubes, appears to correlate with their in vivo myotoxicity (36). This study analyzed in which stage of cellular differentiation of C2C12 myoblasts into myotubes BaspCol-PLA2 was more cytotoxic. Higher susceptibility of myotubes was clearly observed, as originally described by Angulo and Lomonte (36), using a number of myotoxic PLA2s and PLA2 homologues from crotalid venoms. Similar findings were obtained with other bothropic PLA2s such as BmjeTX-I and II (28), and Bj-V from B. jararacussu (37). This fact is probably not only due to the ability of these enzymes to disturb the membrane, but it also seems to be involved with the expression acceptors/ receptors with high affinity for the PLA2s on plasmatic membrane of myotubes. This process could take place when differentiation of myoblast in myotubes is performed. In addition, it is still not possible to state which among the acceptors/receptors or the specific region of PLA2s are involved, or even the type of interactions implicated in the process (3, 33).
BaspCol-PLA2 also induced a moderate edema with highest effect two hours after its injection. Similar results were obtained with other D49 from Bothrops venoms, such as BmjeTx-I and II from B. marajoensis (28) On the other hand, different results were obtained with Myotoxin I, BmTx-I, and BbTx-III from Costa Rican B. asper, B. moojeni, and B. brazili venoms, respectively, whose maximum edema-inducing activity was one hour after their injection (22, 26, 38). Edema caused by Asp49 PLA2s may be due to their combined effect to hydrolyze phospholipid membrane (resulting in the loss of membrane integrity), as well as their metabolic activity generating pro-inflammatory products such as eicosanoids, whose function is to amplify the inflammatory event (39). However, it has been shown that enzymatic inhibition of PLA2s do not completely abolish their edema-forming activity (34), similarly to the myotoxic effect, and as proposed Kini and Evans (35). This suggests the presence of molecular regions that are also responsible for inducing this effect.
Depending on their anticoagulant potency, PLA2 enzymes have been classified into strong, weak and non-anticoagulant enzymes. Strong anticoagulant PLA2 enzymes inhibit blood coagulation at low concentrations (<2 μg/mL). Weak anticoagulant PLA2 enzymes showed anticoagulant effects between 3 and 10 μg/mL (23). BaspCol-PLA2 caused delay of clot formation at doses of 3.1μg/mL or higher. Therefore, this enzyme was classified as weak anticoagulant PLA2. Early PLA2 studies suggested that catalytic activity is essential for anticoagulant effect (40); however, recent studies have proposed that strong anticoagulant PLA2s inhibit by both enzymatic and non-enzymatic mechanisms. The latter are mediated by an ''anticoagulant site'', which would be located in a region between residues 54 and 77, considering that this region is positively charged in the PLA2s with high anticoagulant activity. In PLA2s with moderate or low anticoagulant activity, there is a predominance of negative or neutral charges in this region (23, 41).
The main limitation of this study was the lack of total sequence of BaspCol-PLA2. Thus, we need to perform further studies to obtain total sequence in order to find the regions with highest and lowest identity regard to other snake venom PLA2s.
CONCLUSION
A basic PLA2 was isolated from Colombian B. asper venom; it has catalytic activity and molecular mass of 13974.6 Da. The toxin showed myotoxic, edema-forming, anticoagulant and cytotoxic activities. The activities displayed by this toxin are involved in the local effects observed in Colombian B. asper snakebites. However, molecular mechanisms involved in these effects remain unknown, and further studies of this myotoxin may provide more elements to obtain a better understanding of toxic effects induced by B. asper venom. In addition, this work gave insights about the composition of B. asper venom from Colombia that contains toxins with similar toxic and biochemical profile of those previously reported for toxins isolated from B. asper in other countries.
Conflicts of interest
The authors declare no conflicts of interest.
Financial support
This Project was sponsored by Universidad de Antioquia, project CIQF-177 and Estrategia para la Sostenibilidad de los Grupos de Investigación 2013-2014.
REFERENCES
1. Williams D, Gutiérrez JM, Harrison R, Warrell DA, White J, Winkel KD, et al. The Global Snake Bite Initiative: an antidote for snake bite. Lancet. 2010 Aug 24; 74 (9): 1735-1767. [ Links ]
2. Otero-Patiño R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009; Dec 1; 54 (7): 998-1011. [ Links ]
3. Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003; Dec 15; 42 (8): 827- 840. [ Links ]
4. Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000; Oct 31; 1488 (1-2): 1-19. [ Links ]
5. Arni RK, Ward RJ. Phospholipase A2-a structural review. Toxicon. 1996; Aug; 34 (8): 827-841. [ Links ]
6. Lomonte B, Angulo Y, Calderón, L. An overview of Lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003; Dec 15; 42 (8): 885-901. [ Links ]
7. Ferlan I, Gubensek F. Phospholipases of Bothrops asper venom. Period Biol. 1978; 80: 31-36. [ Links ]
8. Alagón AC, Molinar RR, Possani LD, Fletcher PLJr, Cronan JEJr, Julia JZ. Venom from the snake Bothrops asper Garman. Purification and characterization of three phospholipases A2. Biochem J. 1980; Mar 1; 185 (3): 695-704. [ Links ]
9. Fernández J, Gutiérrez JM, Angulo Y, Sanz L, Juárez P, Calvete JJ, et al. Isolation of an acidic phospholipase A2 from the venom of the snake Bothrops asper of Costa Rica: biochemical and toxicological characterization. Biochimie. 2010; 2010 Mar 1; 92 (3): 273-283. [ Links ]
10. Gutiérrez JM, Ownby CL, Odell GV. Isolation of a myotoxin from Bothropsasper venom: partial characterization and action on skeletal muscle. Toxicon. 1984 Jan 1; 22 (1): 115-128. [ Links ]
11. Lomonte B, Gutiérrez JM. A new muscle-damaging toxin, myotoxin II, from the venom of the snake Bothrops asper (terciopelo). Toxicon. 1989 Jul 1; 27 (7): 725-733. [ Links ]
12. Kaiser II, Gutierrez JM, Plummer D, Aird SD, Odell GV. The amino acid sequence of a myotoxic phospholipase from the venom of Bothrops asper. Arch Biochem Biophys. 1990 May 1; 278 (2): 319-325. [ Links ]
13. Díaz C, Lomonte B, Zamudio F, Gutiérrez JM. Purification and characterization of myotoxin IV, a phospholipase A2 variant, from Bothrops asper snake venom. Nat Toxins.1995 May 1; 3 (1): 26-31. [ Links ]
14. Mebs D, Samejima Y. Isolation and characterization of myotoxic phospholipases A2 from crotalid venoms. Toxicon. 1986 Feb 1; 24 (2): 161-168. [ Links ]
15. Alape-Girón A, Sanz L, Escolano J, Flores-Díaz M, Madrigal M, Sasa M, et al. Snake venomics of the lance head pitviper Bothrops asper: geographic, individual, and ontogenetic variations. J Proteome Res. 2008 Aug; 7 (8): 3556-3571. [ Links ]
16. Segura A, Herrera M, Villalta M, Vargas M, Uscanga-Reynell A, de León-Rosales SP, et al. Venom of Bothrops asper from Mexico and Costa Rica: intraspecific variation and cross-neutralization by antivenoms. Toxicon. 2012 Jan; 59 (1): 158-162. [ Links ]
17. Gutiérrez JM, Escalante T, Rucavado A. Experimental pathophysiology of systemic alterations induced by Bothrops asper snake venom. Toxicon. 2009 Dec 1; 54 (7): 976-987. [ Links ]
18. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15; 227 (5259): 680-685. [ Links ]
19. Cho W, Kézdy FJ. Chromogenic phospholipase A2 substrates and assays. Methods Enzymol. 1991 Sept; 197: 75-79. [ Links ]
20. Holzer M, Mackessy SP. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon. 1996 Oct; 34 (10): 1149-1155. [ Links ]
21. Lomonte B, Angulo Y, Rufini S, Cho W, Giglio JR, Ohno M, et al. Comparative study of the cytolytic activity of myotoxic phospholipases A2 on mouse endothelial (tEnd) and skeletal muscle (C2C12) cells in vitro. Toxicon. 1999 Jan; 37 (1): 145-158. [ Links ]
22. Gutiérrez JM, Lomonte B, Chaves F, Moreno E, Cerdas L. Pharmacological activities of a toxic phospholipase A2 isolated from the venom of the snake Bothrops asper. Comp BiochemPhysiol C. 1986 Jan; 84 (1): 159-164. [ Links ]
23. Kini RM. Structure–function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon. 2005 Jun 15; 45 (8): 1147-1161. [ Links ]
24. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11; 22 (22): 4673-4680. [ Links ]
25. Núñez V, Arce V, Gutiérrez JM, Lomonte B. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops atrox. Toxicon. 2004 Jul; 44 (1): 91-101. [ Links ]
26. Huancahuire-Vega S, Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Structural and functional characterization of brazilitoxins II and III (BbTX-II and -III), two myotoxins from the venom of Bothrops brazili snake. Toxicon. 2009 Nov; 54 (6): 818-827. [ Links ]
27. Pereañez JA, Núñez V, Huancahuire-Vega S, Marangoni S, Ponce-Soto LA. Biochemical and biological characterization of a PLA2 from crotoxin complex of Crotalus durissus cumanensis. Toxicon. 2009 Apr; 53 (5): 534-542. [ Links ]
28. Ponce-Soto LA, Martins-de-Souza D, Marangoni S. Neurotoxic, myotoxic and cytolytic activities of the new basic PLA2 isoforms BmjeTX-I and BmjeTX-II isolated from the Bothrops marajoensis (Marajó Lancehead) snake venom. Protein J. 2010 Feb; 29 (2): 103-113. [ Links ]
29. Arce V, Brenes F, Gutiérrez JM. Degenerative and regenerative changes in murine skeletal muscle after injection of venom from the snake Bothrops asper: a histochemical and immunocytochemical study. Int. J. Exp. Pathol.1991 Apr; 72 (2): 211-226. [ Links ]
30. Gutiérrez JM, Arroyo O, Bolaños R. Mionecrosis, hemorragia y edema inducidos por el veneno de Bothrops asper en ratón blanco. Toxicon.1980 Sept; 18 (5-6): 603-610. [ Links ]
31. Otero R, Osorio RG, Valderrama R, Giraldo AC. Efectos farmacológicos y enzimáticos de venenos de serpientes de Antioquia y Chocó (Colombia). Toxicon. 1992 May; 30 (5-6): 611-620. [ Links ]
32. Lomonte B, León G, Angulo Y, Rucavado A, Núñez V. Neutralization of Bothropsasper venom by antibodies, natural products and synthetic drugs: contributions to understanding snakebite envenomings and their treatment. Toxicon. 2009 Dec 1; 54 (7): 1012-1028. [ Links ]
33. Gutiérrez JM, Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003 Dec 15; 42 (8): 915-931. [ Links ]
34. Soares AM, Giglio JR. Chemical modifications of phospholipases A2 from snake venoms: effects on catalytic and pharmacological properties. Toxicon. 2003 Dec 15; 42 (8): 855-868. [ Links ]
35. Kini RM, Evans HJ. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 1989 Jun; 27(6): 613-635. [ Links ]
36. Angulo Y, Lomonte B. Differential susceptibility of C2C12 myoblasts and myotubes to group II phospholipase A2 myotoxins from crotalid snake venoms. Cell Biochem Funct. 2005 Sept; 23 (5): 307-313. [ Links ]
37. Bonfim VL, Ponce-Soto L, Novello JC, Marangoni S. Cytotoxic Action in Myoblasts and Myotubes (C2C12) and Enzymatic Characterization of a New Phospholipase A2 Isoform (Bj-V) from Bothrops jararacussu Venom. Protein Pept Lett. 2006 jul; 13 (7): 707-713. [ Links ]
38. Calgarotto AK, Damico DC, Ponce-Soto LA, Baldasso PA, Da Silva SL, Souza GH, et al. Biological and biochemical characterization of new basic phospholipase A2 BmTX-I isolated from Bothrops moojeni snake venom. Toxicon. 2008 Jun 15; 51 (8): 1509-1519. [ Links ]
39. Teixeira CF, Landucci EC, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003 Dec 15; 42 (8): 947-962. [ Links ]
40. Verheij HM, Boffa MC, Rothen C, Bryskaert MC, Verger R, De Haas G. Correlation of enzymatic activity and anticoagulant properties of phospholipase A2. Eur J Biochem.1980 Nov; 112 (1): 25-32. [ Links ]
41. Kini RM, Evans HJ. Structure–function relationships of phospholipases. The anticoagulant region of phospholipases A2. J BiolChem. 1987 Oct 25; 262-(30): 14402-14407. [ Links ]