Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.21 no.2 Medellín May/Aug. 2014
ALIMENTOS: CIENCIA, TECNOLOGÍA E INGENIERÍA
ANTIMICROBIAL ACTIVITY OF PROPOLIS AND ITS EFFECT ON THE PHYSICOCHEMICAL AND SENSORAL CHARACTERISTICS IN SAUSAGES
ACTIVIDAD ANTIMICROBIANA DEL PROPÓLEOS Y EFECTO EN LAS CARACTERÍSTICAS FISICOQUÍMICAS Y SENSORIALES EN CHORIZOS
Carolina GUTIÉRREZ-CORTÉS, MSc, PhD(c).1*, Héctor SUAREZ MAHECHA, PhD.1
1 Instituto de Ciencia y Tecnología de Alimentos - ICTA, Universidad Nacional de Colombia sede Bogotá. Carrera 30 No. 45-03 edificio 500 C. Bogotá Colombia.
* Autor a quien se debe dirigir la correspondencia: cgutierrezco@unal.edu.co.
Received: 08 Novembre 2012
Accepted: 26 May 2014
ABSTRACT
Background: In the food industry, the use of natural additives capable of replacing chemical additives is increasing due to the tendency to consume natural and healthy foods. In nature, different substances such as propolis can fulfill that role and it is obtained from hives of honey bees. Propolis contains bioactive compounds with antimicrobial and antioxidant capacity, and thus, they could be used as an alternative for nitrites in meat products. Objective: To evaluate the in-vitro antimicrobial activity of the ethanol propolis extract (EEP) of on some pathogenic bacteria and their influence on the physicochemical and sensorial properties of sausages. Methods: The ethanol extraction was performed, and its antimicrobial activity in vitro on S. aureus, Salmonella, E. coli and Clostridium spp. was determined. Sausages were prepared according to the following treatments: (1) EEP 0.8mg/mL, (2) 0.2g/Kg sodium nitrite and sodium erythorbate, (3) Alcohol (96% v/v used as control) and the physicochemical analysis was performed to determine thiobarbituric acid (TBA), volatile nitrogen bases (TVB - N) and sensoral tests every week for four weeks. Analysis of variance and Tukey test were performed and the level of significance was p < 0.05. Results: No significant differences between EEP concentration, pH and TVB-N or sensorial properties (p > 0.05) were found. However, significant differences in TBA values were observed (p < 0.05). Conclusions: A 0.8% EEP shows antimicrobial activity against some pathogenic microorganisms evaluated. Further, the physicochemical and sensorial characteristics of the product do not differ from the sausages containing nitrites.
Keywords: Propolis, antioxidant, decarboxylation, sensorial characteristics, sausage.
Resumen
Antecedentes: En la industria de alimentos es cada vez más común la utilización de aditivos naturales capaces de reemplazar los aditivos químicos, esto es debido a la tendencia a consumir alimentos más naturales y saludables. En la naturaleza existen diferentes compuestos que pueden cumplir dicha función, como el caso de los propóleos obtenidos en las colmenas de las abejas melíferas que presentan compuestos bioactivos con capacidad antimicrobiana y antioxidante y, por tanto, podría presentarse como una alternativa a la utilización de nitritos en productos cárnicos. Objetivo: Valorar la actividad antimicrobiana in-vitro del extracto etanólico de propóleos (EEP) sobre ciertas bacterias patógenas y su influencia en las características fisicoquímicas y sensoriales de chorizos. Métodos: Se realizó la extracción de los propóleos con alcohol etanólico al 96% y se determinó su actividad antimicrobiana in vitro sobre S. aureus, Salmonella spp., E. coli y Clostridium spp. Se prepararon chorizos de acuerdo a los siguientes tratamientos: (1) EEP 0.8mg/mL; (2) 0,2g/Kg de nitrito de sodio y eritorbato de sodio; (3) alcohol 96% (control) y se realizaron los análisis fisicoquímicos correspondientes a la determinación de ácido tiobarbitúrico (TBA) y bases volátiles de nitrógeno (BVT-N) y pruebas sensoriales cada ocho días durante cuatro semanas. Se realizó análisis de varianza de dos vías y prueba de Tukey, el nivel de significancia fue de p<0,05. Resultados: No se encontraron diferencias significativas entre las concentraciones de EEP en la actividad antimicrobiana in vitro, en los valores pH y BVT-N ni en la evaluación sensorial (p>0,05). Se observaron diferencias significativas (p<0,05) en los valores de TBA. Conclusiones: El EEP al 0,8% presenta actividad antimicrobiana para los microorganismos patógenos evaluados. Adicionalmente, las características fisicoquímicas y sensoriales del producto no difieren de los chorizos elaborados con nitrito.
Palabras clave: Propóleos, antioxidante, descarboxilación, características sensoriales, chorizo.
INTRODUCTION
A Chorizo is a meat product (sausage) of massive consumption in Colombia, it is made from bovine and porcine meat, porcine fat, and other species. According to the Colombian Technical Standard (NTC) 1325 sausages must have not more than 200 mg/Kg of nitrite, which means that nitrite is still used in low concentrations as preservative and color additive in the sausages commercialized in the country.
Lipid autoxidation is a chemical change that can decrease the quality of the meat products because it generates undesirables odor, color, smell, and flavor reducing the nutritional value (1). Another quality indicator of this meat product is the amount of nitrogen volatile bases (TVB-N) such as ammonium, thrimethylamine, and dimethilamine. The increase in the amount of TVB-N is associated with spoilage, either from bacterial action or by endogenic enzymes, and therefore it is an indicative of food freshness (2). The bacterial contamination decreases the quality of the product and also is a present danger to the consumer health.
Some additives used as antioxidants and preservatives have proved be unhealthy to the consumers. Therefore, it is important to develop natural additives such as sodium nitrite, which has been commonly used as a preservative since it acts as an antioxidant agent, reducing agent and nitrosylant. Nitrite is converted into different compounds as nitrous acid, nitric oxide, and nitrate (2, 3). Nitric oxide is formed when nitrite reacts with the sulfhydryl groups of proteins, and it can inhibit the growing of Clostridium botulinum. Nevertheless, it is known that nitrites react with the biogen amines produced for the decarboxilation of some aminoacids (5), producingnitrogenated compounds known as cancer precursors (6).
Alternatively, there are numerous natural alternatives substances as antimicrobial agents. For example, essential oils obtained from aromatic plants that can inhibit bacterial growth (4-6), chitosan, which is obtained from the exoskeleton of insects and crustaceans also has bactericidal activity (7, 8). Further, bacteriocins, which are secondary metabolites produced by certain lactic bacteria, have been used in the food industry (9-11), and propolis, which is extracted from hives of honeybees, has been used in some meat products (12-15). Propolis is an important and promising natural substitute for antimicrobial agents.
Propolis is a natural product obtained from honeybees composed from resins collected from plant secretions in bulbs and leaves and is used to cover hives and protect them (16). Propolis is composed of 45% resins, 30% wax and 10% fatty acids of essential oils, 5% pollen, and 10% organic compounds and minerals. During the extraction with ethanol the wax and the organic waste are removed to obtain the ethanolic extract of propolis (EEP) with all the bioactive constituents. More than 300 components have been isolated, such as terpenoids, flavonoids, phenolic acids, steroids, sugars, amino acid, among others. Propolis is known as an antioxidant, antibacterial, antifungal, antiviral, anti-inflammatory, antitumoral agent. It is also hepatoprotective, local anesthetic, inmunoestimulator, and antimutagenic (20). Numerous studies have demonstrated the antimicrobial activity of EEP with different concentrations.
The aim of this work is the evaluation of the antioxidant characteristics of propolis used in the sausages production, and to verify the amount of volatile nitrogen bases as indicator of the microbial contamination of the products and compare these results with the sensorial evaluation.
MATERIALS AND METHODS
Ethanolic extract of propolis (EEP)
100 g of propolis obtained in Boyacá - Colombia were introduced in a precipitated glass and 400 mL of a 96% ethanol were added. The mixture was shaken during two hours, and it was allowed to stand overnight and filtered. The residue was subjected to a secondary extraction under the same conditions. Finally, the two extracts were mixed and frozen to precipitate other compounds. The supernatant EEP was used for the tests and contained an 8% of total solids.
In-vitro diffusion test
The antimicrobial activity of EEP was analyzed by agar diffusion test (Mueller-Hinton). Three different concentrations were used in order to know which onecould be used in the formulation of the sausages. The microorganisms used were Staphylococcus aureus, Escherichia coli, Salmonella spp. and Clostridium sp. These strains were obtained from the laboratory of the Institute of Food Science and Technology, (ICTA) National University of Colombia in Bogotá. The activation of the strains was performed in the BHI broth (Oxoid), TSB (Merck) and nutritive broth (Oxoid). They were incubated overnight and then cultivated in BHI a broth. 1 mL of each strain was inoculated using the 0.5 McFarland scale (concentration corresponding to 1.5 X109 cells) on the surface of the Mueller-Hinton agar. A 6mm diameter disks were impregnated with three different concentrations of propolis (0.8, 1.2, and 1.6 mg/mL) and a negative control with 96%. alcohol was conducted. They were incubated for 48 hours at 37oC and the inhibition zones were measured. Clostridium sp. was incubated in anaerobic conditions. The test was made in duplicate.
Production of fresh sausages
Fresh sausages of 100 g each were made with porcine meat (60%), bovine meat (20%), and porcine fat (20%). Three treatments were made using the same formulation; the only difference was the preservative content: T1) 0.8 mg/mL EEP; T2) 0.2 g/kg sodium nitrite and 0.5 g/kg sodium erythorbate; and T3) 96% alcohol as control.
Meat and fat were cut into pieces and minced using a cutter equipped with 12 mm discs and separated into three parts to mix with the other ingredients (pepper, garlic, salt, cayenne, scallions). The respective preservative was added to each treatment and the sausages were stuffed into pork casings, tied and packaged in sealed bags under vacuum stored in the meat plant of ICTA at a temperature of 50oC and at 89 - 93% relative humidity.
Physicochemical analysis
pH
The pH measurement was performed using a potentiometer (Schott - Handylab), samples were passed through a manual meat grinder (Premier MG-1724) with a mesh opening of 5 mm in diameter. Three different measurements were made for each sample, directly.
Determination of total volatile nitrogen bases (TVB-N)
10 g of sample, 2 g of magnesium oxide, and 150 mL of distillated water were added in a distillation flask. The mixture was distillated and collected in a 3% boric acid solution to complete ~100 mL of volume. Three drops of the Tashiro indicator were added to the distillate. Finally, the distillate was titrated with 0.1 N HCl (17).
The amount of TVB-N in mg/100 g of sample was calculated from the volume (V) of the added hydrochloric acid and its concentration (C) using the following equation (1):

Thiobarbituric acid (TBA) determination
Approximately, 10 g of sausage was mixed with 50 mL of distilled water in a blender. The mixture was transferred onto a distillation flask, and the residue was washed with 47.5 mL of distillated water, and transferred to the distillation flask. 2.5 mL of 4 N HCl and few drops of antifoam silicone were added. The mixture was distilled for 10 minutes to collect ~50 mL.
5 mL of the distilled product was placed in a test tube and 5 mL of 0.02 M TBA solution was added to the tube. It was capped and heated in a boiling water bath for 35 minutes. The product was transferred to a 1 cm cell to measure the absorbance at 532 nm in a UV-Vis spectrophotometer (Jasco 530). The results were expressed in mg of malondialdehyde Antimicrobial activity of propolis and its effect on the physicochemical and sensoral characteristics in sausages 93 per kilogram in comparison to the calibration curve built with 1,1,3,3-tetramethoxypropane (18). The test was performed on a portion of the distillate from the sample and distilled water treated under the same conditions was used as a negative control.
Sensorial evaluation
The sensorial characteristics of sausages made with propolis and sodium nitrite were compared. The analyses were performed at 0, 8, 16, and 24 days. Sausages were submitted to a panel of 10 trained tasters in order to evaluate the sensorial attributes. The panelists were selected from the staff members of the Institute of Food Science and Technology (ICTA) taking into account their habits, expertise with sausages, their sensitivity and their ability to reproduce the evaluations. Prior to testing, the panelists were trained in the sensorial vocabulary related to meat products. Further, the panelists were trained to know the features of the procedure. Samples were coded and presented to all panel members in a random order (19).
Sausages were cooked and fried for 20 minutes and then were cut into slides. The sensorial evaluation was divided in two parts. First, uncooked samples were tested to qualify odor and color. Then, panelists were asked to indicate taste, odor, and color attributes of the cooked sausages in this order. The test of cooked samples corresponding to odor and taste attributes was made blindfolding. Unsalted crackers and water at room temperature were also provided to clean the palate between sampling. Panelists were blindfolded to prevent the red color from interfering with their judgment. Finally, the color test was made. The Hedonic scale of nine points was used for the attributes, where 1 corresponded to extremely unpleasant, 9 to extremely pleasant and 4 to satisfactory (10).
Statistical Analysis
Data from physicochemical and sensory test were analyzed with a two ways ANOVA for two factor design with interactions. It was made the multiple comparison among the treatments with Tukey´s test (P<0.05) using the R program version 2.12.2
RESULTS
In - vitro diffusion tests
EEP concentrations of 0.8, 1.2 and 1.6 mg/ mL showed inhibition of the pathogenic bacteria. No significant differences (p> 0.05) among the diameters of the inhibition zones presented by the different concentrations of ethanol extract of propolis against Salmonella spp., E coli, S. aureus and Clostridium sp., was found at the lowest concentration (0.8mg/mL).
Physicochemical analysis
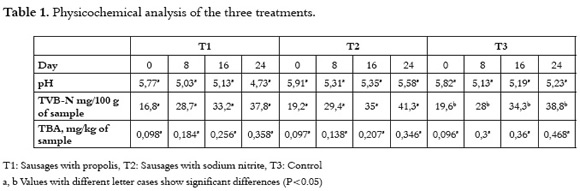
Table 1 shows the pH, volatile nitrogen bases (TVB-N), and thiobarbituric acid (TBA) results. Acid values decreased from the eight day of storage and increased again after 24 days. The pH T1 treatment presented the lowest values. TVB-N increased in the three treatments; however, the T1treatment showed lower values during the whole period of study. The TBA also increased in the three treatments, where the T2 treatment presented the lowest values, followed by the T1treatment.
Figure 1 shows the pH values during the 24 days study. In all cases, the pH decreased up to the 8th day which suggests the presence of acid lactic bacteria, which were able to grow under anaerobic conditions generated by the vacuum packaging. This development produce lactic acid, which was responsible for the pH reduction (20). From the 16 day the pH started to increase in T2 and T1, due to the enzymatic denaturalization which occurs during the storage period.
An increase in the TVB-N was observed (Figure 2). T1 presented the lower amount among the three treatments. A correlation between the increase of TVB-N with the increase of pH in day 16 for T1 and T2 was observed. It suggests that the presence of microorganisms could remain until the final period of storage and participate in the oxidative decarboxylation.
The amount of TBA is related to the antioxidant activity of propolis. Further, TBA values showed that lipid oxidation increased (Figure 3) until day 24 and significant differences among treatments (P<0.05) were observed. T1 and T2 showed a lower increment as compared to the control, demonstrating the antioxidant activity of propolis reported by other authors (21, 22). T1 presented the slowest increase, followed by T2.
Sensorial evaluation
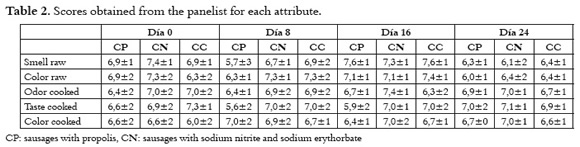
Panelists showed their preference for the traditional red color. However, this was not a reason to reject the raw or cooked product during the test. Taste was qualified good in the cooked product and the panelists considered that the appearance and odor were good. For this reason, most of the scores were higher than four, and were considered within the acceptation range.
Table 2 shows the scores for each treatment in the sensorial evaluation. No significant differences were found in the sensorial evaluation among the treatments (P>0.05). A scale from 1 to 9 was used, where the scores higher than 4 were considered within the acceptation criteria by the panelists. Color and taste attributes improved with time. However, the statistical test does not showed any difference.
DISCUSSION
Inhibition zones higher than 8 mm in diameter were obtained; it agreed with a study done in different regions of Argentina which reported an antimicrobial activity of EEP on S. aureus with inhibition zones higher than9 mm diameter, which were considered having a significant antimicrobial activity (21).
In a previous study, the method of viable cell count in a TCBS agar with different concentrations of EEP obtained from three regions of Taiwan in a four month period of sampling. They reported a bactericidal activity at concentrations from 0.75 to 1.2 mg/mL depending on the site and month of collection. According to that report, the concentration of EEP used in the sausages (0.8mg/mL) is included within the range (13). It has been reported that EEP concentrations of 1.25 mg/mL has an inhibitory activity for Gram positive and a 5 mg/mL EEP concentration for Gram negative bacteria (23). It suggests that using higher EEP concentrations make the inhibition possible in a broader spectrum.
In another study the minimum inhibitory concentration (MIC) testing different concentrations of EEP against bacteria and filamentous fungi determined that the antimicrobial activity is achieved in a concentrations range from 0.005 to 0.5 mg/ mL. The most resistant bacteria were E. coli and Micrococcus luteus (24). This concentration was lower than the one reported by another study in which it was 5 mg/mL inhibiting the growth of E. coli, 2.5 mg/mL inhibiting Salmonella typhymurium, while S. aureus was inhibited with a 1.5 mg/mL of EEP (19). The information above suggests that a low concentration of propolis decrease the microbial growth responsible for food spoilage and can be related with meat product preservation.
In other studies, the reported initial pH of raw material for sausages was between 5.4 and 5.5. This acid value is due to the post-mortem glycolysis generated by the in-situ proteolytic and lipolytic enzymes of the meat (25). The microbial activity can also generate acid conditions. The reported values of pH are very close to those obtained in this work in the first day of the study. The observed behavior matches the ones proposed by other authors (27). This is the result of the action of decarboxylase enzymes from microorganisms present in meat on proteins, and in a low proportion by the endogenic enzymes present in the muscle. No significant differences were found in the three treatments (P>0.05). After 24 days, T1 presented a 37.8 TVB-N mg/100, this value was larger than the one reported by (12) after 21 days of storage (19.3 TVB-N mg/100 g).
The TBA results showed low values in contrast with previous studies (26), where authors reported values of 16.83 and 6.07 mg of malondialdehyde (MDA)/Kg for bovine and pig meat respectively, after ten days of storage. In the present study, 0.358 mg MDA/Kg after 24 days of storage was observed. Further, 0.89 mg MDA/ Kg after 21 days of storage in sausages is reported in (12). The reported main compounds responsible of the antioxidant action of propolis from Greece and Cyprus, were phenolic acids, anthraquinones, and flavonoids (19). On the other hand, polyphenols are the main compounds present in propolis from China, with an antioxidant capacity (27).
Panelists commented on the different color as a feature which does not disqualify the product, but it could be improved due to the difference with the color of traditional sausage. According to the survey (28), it is possible to use the natural sources of nitrite as celery or introduce nitrate-reducing microorganisms which may help the color formation.
These can be the acid lactic bacteria, which can act as starter in fermentation of meat products (29) and are resistant to the antimicrobial action of propolis (19). Finally, a mixture, in which is possible to take advantage of the antioxidant and antimicrobial capacities of propolis, would be obtained retaining most of the traditional organoleptic characteristics. In respect to taste, most consumers did not detect differences and did not have suggestions.
Propolis showed similar behavior to nitrites when was applied in a meat product by controlling the acidification of the product and the production of TBA and TBV-N. This presents a great opportunity to replace sodium nitrite as a preservative for meat products. Antimicrobial and antioxidant activities of propolis have been studied, but few studies have been focused on the implementation and evaluation of products.
Propolis shows a huge potential in the food industry, but it has a limitation for use because its preparation and extraction can be expensive and therefore, this procedure should be optimized to increase their availability. Further, in a future work it can be used to test higher concentrations of propolis to perform microbiological testing to verify counts over time.
CONCLUSIONS
The concentration of EEP used in the preparation of sausages showed an antioxidant capacity similar to the sodium nitrite used traditionally as a curing agent and preservative. Propolis is able to control the formation of TVB-N, for this reason, it is assumed its usefulness on the growth of the microorganisms and also retarded the protein degradation. Sensorial characteristics of the sausages with EEP are accepted by the consumers; however, it is suggested to improve the color. In vitro results show a great ability of propolis to inhibit microbial growth; nevertheless it is important to develop a microbiological analysis in order to know the behavior of the bacterial population within the products. Propolis has been proved as an alternative for natural preservation of meat products. The shortcoming of the red color can be solved by using plant colorants. It is necessary to develop a better protocol of EEP extraction because this procedure is complicated and extensive.Furthermore, it is important to consider possible difficulties when propolis is collected.
REFERENCES
1. Fernández J, Pérez-Álvarez JA, Fernández-López JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chemistry. 1997; 59(3): 345-53. [ Links ]
2. Honikel K-O. The use and control of nitrate and nitrite for the processing of meat products Meat Science. 2008; 78(1-2): 68-76. [ Links ]
3. Pegg RB, Shahidi F. Nitrite curing of meat: the N-nitrosamine problem and nitrite alternatives: Food & Nutrition Press; 2004. [ Links ]
4. Duarte MCT, Leme EE, Delarmelina C, Soares AA, Figueira GM, Sartoratto A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. Journal of Ethnopharmacology. 2007; 111(2): 197-201. [ Links ]
5. Lv F, Liang H, Yuan Q, Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Research International. 2011; 44(9): 3057-64. [ Links ]
6. Rojas-Graü MA, Avena-Bustillos RJ, Olsen C, Friedman M, Henika PR, Martín-Belloso O, et al. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate-apple puree edible films. Journal of Food Engineering. 2007; 81(3): 634-41. [ Links ]
7. Fernandez-Saiz P, Lagaron JM, Ocio MJ. Optimization of the biocide properties of chitosan for its application in the design of active films of interest in the food area. Food Hydrocolloid. 2009; 23(3): 913-21. [ Links ]
8. Gómez-Estaca J, López de Lacey A, López-Caballero ME, Gómez-Guillén MC, Montero P. Biodegradable gelatin-chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiology. 2010; 27(7): 889-96. [ Links ]
9. Belfiore C, Castellano P, Vignolo G. Reduction of Escherichia coli population following treatment with bacteriocins from lactic acid bacteria and chelators. Food Microbiology. 2007; 24(3): 223-9. [ Links ]
10. Suárez H., Pardo S., M. C. Calidad físico-química y atributos sensoriales de filetes sajados biopreservados de cachama, empacados al vacío bajo refrigeración. Revista colombiana de ciencias pecuarias. 2008; 21: 330-9. [ Links ]
11. Wijnker JJ, Weerts EAWS, Breukink EJ, Houben JH, Lipman LJA. Reduction of Clostridium sporogenes spore outgrowth in natural sausage casings using nisin. Food Microbiology. 2011; 28(5): 974-9. [ Links ]
12. Ali FH, Kassem GM, Atta-Alla OA. Propolis as a natural decontaminant and antioxidant in fresh oriental sausage. La propoli come decontaminante e antiossidante nella salsiccia orientale fresca. 2010; 46(2):167-72. [ Links ]
13. Lu L-C, Chen Y-W, Chou C-C. Antibacterial activity of propolis against Staphylococcus aureus. International Journal of Food Microbiology. 2005;102(2): 213-20. [ Links ]
14. Orsi RO, Sforcin JM, Rall VLM, Funari SRC, Barbosa L, Fernandes JA. Susceptibility profile of Salmonella against the antibacterial activity of propolis produced in two regions of Brazil. Journal of Venomous Animal Toxins. 2005;11: 109-16. [ Links ]
15. Tolosa L, Cañizares, E. Obtención, caracterización y evaluación de la actividad antimicrobiana de extractos de propóleos de Campeche. ARS Pharmaceutical. 2002:187-204. [ Links ]
16. Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chemistry. 2009;116(2): 452-61. [ Links ]
17. ICONTEC. NTC 1322. Productos de la pesca. Métodos de análisis físicos y químicos. 2007; Instituto Colombiano de Normas Técnicas y Certificación:[Segunda actualización Editada 2007-12-2] [ Links ]
18. Tarladgis B, Watts B, Younathan M, Dugan L, Jr. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960; 37(1): 44-8. [ Links ]
19. Duthoit F, Callon C, Tessier L, Montel M-C. Relationships between sensorial characteristics and microbial dynamics in ''Registered Designation of Origin'' Salers cheese. International Journal of Food Microbiology. 2005;103(3): 259-70. [ Links ]
20. Krockel L. The Role of Lactic Acid Bacteria in Safety and Flavour Development of Meat and Meat Products. In: Kongo DJM, editor. Lactic Acid Bacteria - R & D for Food, Health and Livestock Purposes. http://www.intechopen.com/books/lacticacid- bacteria-r-d-for-food-health-and-livestock-purposes/therole- of-lactic-acid-bacteria-in-safety-and-flavour-developmentof- meat-and-meat-products. [ Links ]
21. Chaillou LL, Nazareno MA. Bioactivity of propolis from Santiago del Estero, Argentina, related to their chemical composition. LWT - Food Science and Technology. 2009; 42(8): 1422-7. [ Links ]
22. Choi YM, Noh DO, Cho SY, Suh HJ, Kim KM, Kim JM. Antioxidant and antimicrobial activities of propolis from several regions of Korea. LWT - Food Science and Technology. 2006; 39 (7): 756-61. [ Links ]
23. Mohammadzadeh S, Shariatpanahi M, Hamedi M, Ahmadkhaniha R, Samadi N, Ostad SN. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem. 2007; 103(4): 1097-103. [ Links ]
24. Garedew A, Schmolz E, Lamprecht I. Microbiological and calorimetric investigations on the antimicrobial actions of different propolis extracts: an in vitro approach. Thermochimica Acta. 2004; 422 (1-2): 115-24. [ Links ]
25. Nychas G-JE, Skandamis PN, Tassou CC, Koutsoumanis KP. Meat spoilage during distribution. Meat Science. 2008;78 (1-2): 77-89. [ Links ]
26. Tang S, Kerry JP, Sheehan D, Buckley DJ, Morrissey PA. Antioxidative effect of added tea catechins on susceptibility of cooked red meat, poultry and fish patties to lipid oxidation. Food Research International. 2001; 34 (8): 651-7. [ Links ]
27. Ahn M-R, Kumazawa S, Usui Y, Nakamura J, Matsuka M, Zhu F, et al. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chemistry. 2007; 101 (4): 1383-92. [ Links ]
28. Sebranek JG, Bacus JN. Cured meat products without direct addition of nitrate or nitrite: what are the issues? Meat Science. 2007; 77 (1):136-47. [ Links ]
29. Zhang X, Kong B, Xiong YL. Production of cured meat color in nitrite-free Harbin red sausage by Lactobacillus fermentum fermentation. Meat Science. 2007; 77 (4): 593-8. [ Links ]