Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.21 no.3 Medellín Sept./Dec. 2014
FOODS: SCIENCE, TECHNOLOGY AND ENGINEERING
THE ADSORPTION THERMODYNAMICS OF SUGARCANE (Saccharum officinarum L.) POWDER OBTAINED BY SPRAY DRYING TECHNOLOGY
TERMODINÁMICA DE ADSORCIÓN DE POLVO DE CAÑA DE AZÚCAR (Saccharum officinarum L.) OBTENIDO MEDIANTE LA TECNOLOGÍA DE SECADO POR ASPERSIÓN
Esteban LARGO-AVILA, MSc; Misael CORTES RODRÍGUEZ, Ph.D*; Héctor J. CIRO-VELASQUEZ, Ph.D
Universidad Nacional de Colombia. Medellín, Colombia.
* Autor a quien se debe dirigir la correspondencia: mcortesro@unal.edu.co.
Recibido: Julio 7 de 2013
Aceptado: Septiembre 18 de 2014
ABSTRACT
Background: Sugarcane is one of the world's largest crop. It grows in the tropical and subtropical regions, and its harvest provides 80% of the world's sugar. In Latin America unrefined cane sugar is widely available and much less expensive than refined sugar. Sugarcane is a crop of great interest in Colombia due to the economic impact on the rural population and its application as sweetener agent. The powder of sugarcane (Saccharum officinarum L.) is widely used as a raw material in a wide range of industries such as foods, pharmaceutical, cosmetic and chemical. Objectives: The aim of the research work was the evaluation of the adsorption thermodynamics of sugarcane powder obtained by spray drying technology. Methods: The adsorption isotherms of sugarcane powder were evaluated at temperatures of 4 ± 0.1, 20 ± 0.2 and 30 ± 0.3 °C and its thermodynamic properties such as Gibbs free energy (G), differential heat of adsorption (ΔH) and differential entropy (ΔS) were calculated as a function of moisture content. Experimental data of adsorption isotherms were fitted to the GAB (Guggenheim - Andersen - de Boer), BET (Brunauer - Emmett - Teller), Henderson, Caurie, Smith, Hasley, Peleg, and Oswin models. Results: The results showed a type-II sigmoidal behavior, with temperature having a statistically significant effect. The GAB equation showed a better fit to the experimental data modeling (0.11≤aw≤0.87) although all models showed validity and goodness of fit to the experimental data. The net isosteric heat increased to a maximum value (57 kJ mol-1) and then decreased with the increase in moisture content. Conclusions: The sugarcane powder with maltodextrin, obtained by spray drying got low adsorption thermodynamic stability, as it required very low energy to occur this phenomenon, being obtained the maximum net isosteric heat when moisture content of 4.7% (d.b). This value is within the range of the monolayer moisture content found in the GAB and BET models.
Keywords: Saccharum officinarum L., isotherms of adsorption, thermodynamics, powders.
RESUMEN
Antecedentes: La caña de azúcar es uno de los cultivos más grande del mundo. Crece en las regiones tropicales y subtropicales, y su cosecha proporciona el 80% del azúcar del mundo. En América Latina, la panela es ampliamente disponible y es mucho menos costoso que el azúcar refinado. La caña panelera es un cultivo de gran interés en Colombia debido a su impacto sobre la economía rural y a sus propiedades edulcorantes. El polvo de caña de azúcar (Saccharum officinarum L.) presenta múltiples usos como ingrediente para la industria alimentaria, farmacéutica, cosmética y química. Objetivos: El objetivo fue evaluar la termodinámica de adsorción del polvo de caña de azúcar obtenido mediante la tecnología de secado por aspersión. Métodos: Las isotermas de adsorción de polvo de caña de azúcar fueron evaluadas a temperaturas de 4 ± 0.1, 20 ± 0.2 y 30 ± 0.3 °C y sus propiedades termodinámicas tales como energía libre de Gibbs (ΔG), calor diferencial de adsorción (ΔH) y entropía diferencial (ΔS) fueron determinadas en función del contenido de humedad. Los datos experimentales de las isotermas de adsorción se ajustaron a los modelos teóricos de GAB (Guggenheim - Andersen - de Boer), BET BET (Brunauer - Emmett - Teller), Henderson, Caurie, Smith, Hasley, Peleg y Oswin. Resultados: Los resultados mostraron un comportamiento sigmoidal de tipo II, con la temperatura como factor de efecto significativo. El modelo GAB mostró el mejor ajuste con relación a los datos experimentales (0,11 ≤ aw ≤ 0,87). Sin embargo, todos los modelos mostraron validez y buena calidad de ajuste con relación a los datos experimentales. El calor neto isostérico aumentó a un máximo valor de 57 kJ mol-1, disminuyendo con el aumento de contenido de humedad del producto. Conclusiones: El polvo de caña de azúcar con maltodextrina obtenido por secado por pulverización presenta poca estabilidad termodinámica a la adsorción, ya que muy poca energía se requiere para que ocurra este fenómeno, siendo el calor isostérico neto máximo a un contenido de humedad de 4,7% (b.s). Este valor está dentro del rango de contenido de humedad de la monocapa estimada a través de los modelos de GAB y BET.
Palabras clave: Saccharum officinarum L., isotermas de adsorción, termodinámica, polvos.
INTRODUCTION
Originally from Southeast Asia, sugarcane (Saccharum officinarum L.) is a perennial grass from the Poaceae family. This plant is grown in tropical countries because the juice of its stem is an important source of saccharose (1). It is used as a source of food, energy (ethanol or biofuel), and for manufactured products such as jaggery. The latter is considered one of the agricultural industries with the longest tradition in Latin America and the Caribbean. The unrefined whole cane sugar industry has approximately 50000 traditional sugarcane mills known as ''trapiches'', and over a million workers (2). Unrefined whole cane sugar has different names throughout South America: in Colombia, it is called ''panela'', in Peru and Chile, ''chancaca'', in Brazil ''rapadura'', in Venezuela, Mexico, and Guatemala, it is known as ''papelón'', and in India, and probably many other Eastern countries, ''jaggery'' or sometimes ''gur'' or ''gul''. The FAO calls it ''noncentrifugal sugar'' (3, 4).
Panela, which is basically a solid block of glucose and fructose obtained from the evaporation of sugarcane juice, is a traditional product in Latin America. The main producer of panela is Colombia (about 1.4 million tons/year). Panela production is one of Colombia's most important economic activities, and has the highest consumption per capita in the world. In Colombia, sugarcane is used mainly for sugar and panela production; the latter, however, is a low-technology agro-industry. There is a very low rate of introduction of new technologies and production costs are high. These issues have delayed expansion towards both internal and international markets (5).
Additional problems affect panela production, such as poor diversification of production, a lack of integrated marketing, inflexible and varied periods of oversupply resulting in price depression, and, finally, a lack of control over some physical, chemical, and microbiological characteristics relating to quality.
This has induced modern consumers to prefer sugar as a sweetener, due to its quick and easy dissolution and the homogeneity of its shape, despite the fact that it is nutritionally inferior, mainly in terms of protein, mineral, and vitamin content (6, 7).
In order to promote its consumption, ground jaggery obtained by mechanical methods has been categorized as a flagship product, taking advantage of the variety of uses that it has as a raw material for the manufacture of other food products (4). The spray drying of sugarcane juice needs to be evaluated, since it offers an alternative presentation of sugarcane juice with the following advantages: stability during storage, ease of handling, instantaneous reconstitution as a beverage (whilst preserving the original characteristics of sugarcane juice), and the fact that it helps reduce packaging, storage, and transportation costs (8).
Understanding water sorption isotherms together with the thermodynamic properties of this type of product provides valuable information about the mechanisms of mass transfer and the energy requirements associated with the sorption behaviour of this food and water (9). These concepts are very important for determining drying, storage, and packaging requirements, as well as for predicting the product's shelf life (10). Several mathematical models have been used to describe the behavior of these kinds of food matrices, and whilst some models are based on theoretical mechanisms, others are purely empirical: BET (Brunauer - Emmett - Teller), GAB (Guggenheim - Andersen - de Boer), Henderson, Caurí, Smith, Hasley, Oswin, Peleg, Chung, Pfost, etc (11 - 14). However, for some agricultural products these models have not been very effective (15, 16).
Knowledge of the total sorption heat or isosteric sorption heat is very useful in order to study sorption processes in foods and when designing equipment for dehydration processes. In these processes, sorption heat represents the energy required to break the intermolecular forces between the water in the environment and on the surface of the food. Likewise, when water adsorption phenomena occur in powdered foods, sorption heat corresponds to the energy needed for the reverse process to occur (9, 17). The Gibbs free energy of sorption is related to the spontaneity of a process (ΔG<0) and sorption entropy may be associated with spatial rearrangements occurring in the adsorbate - adsorbent interface (ΔS<0 describes a more structured system) (10, 14, 18, 19).
Spray drying technology has been used to obtain high-quality powdered food from sugar-rich foods, dairy products, vegetable products, concentrated juices, green tea, amongst others (19 - 26). Spray drying on fruits and vegetables rich in sugars is difficult due to they have both low melting points and glass transition temperature (Tg). Therefore using of maltodextrin (MD) as encapsulant additive has been using in the last years in spray drying, as it increases the Tg, and enhance its proprieties and its stability as well during its storage (27, 28).
By performing a literature review was not found studies about to sorption thermodynamics of sugarcane powder obtained by spray drying, however studies have been carried out on ground panela (29). The objective of this study was the evaluation of the adsorption thermodynamics of sugarcane powder obtained by spray drying technology.
MATERIALS AND METHODS
Location
The experiments were conducted at the Agricultural Processing laboratory and the Food Quality Control laboratory (Department of Agricultural and Food Engineering) at National University of Colombia, Medellín-Campus.
Raw Material
Concentrated sugarcane juice was obtained from a traditional sugarcane mill in a rural area in the Department of Antioquia (Colombia-South America) and stored under refrigeration at 4°C. An auxiliary drying additive consisting of maltodextrin (MD) with equivalent dextrose (DE) of 19-20 (Shandong Boalingbao Biotechnology Co. Ltd.) was used.
Spray Drying Process
The concentrated sugarcane was mixed with maltodextrin and homogenized at 15000 RPM (Ultraturrax T25, IKA) for 5 minutes, and the mixture was filtered using a 500 μm mesh. The following physical and chemical characteristics were evaluated: pH was measured using a pH meter (Hanna pH 211 automatic titrator) by submerging the electrodes in the sample after calibration with buffer solutions whose pH was 4 and 7 at 25°C (AOAC 981.12); total soluble solids content was determined by the index of refraction and expressed as °Brix at 25ºC (AOAC 932.12); acidity was measured by titration with 0.1 N NaOH using phenolphthalein as an indicator and was expressed as percentage of citric acid per 100 g of sample (AOAC 942.15) (30).
The drying process was carried out using a spray dryer with an atomizer disk (Vibrasec S.A. model PSA 1.5) and water evaporation capacity of 1.5 Lh-1, under the following operating conditions: inlet air temperature (130 °C), outlet air temperature (75 °C), atomizer disc speed (22000 rpm), and a feed mass of sugarcane concentrate with 20% maltodextrin. The optimization process was performed using the methodology of response surface with central composite experimental design with four factors: maltodextrin (10 - 20%), inlet air temperature (130 - 150 °C), outlet air temperature (75 - 85 °C) and atomizer disk speed (22000 - 26000 rpm). The following response variables were optimized: effective recovery (maximum), formation of deposits in the drying chamber (minimum), aw (minimum), moisture (minimum), solubility (maximum) and hygroscopicity (minimum) (31).
Sorption isotherms and mathematical models
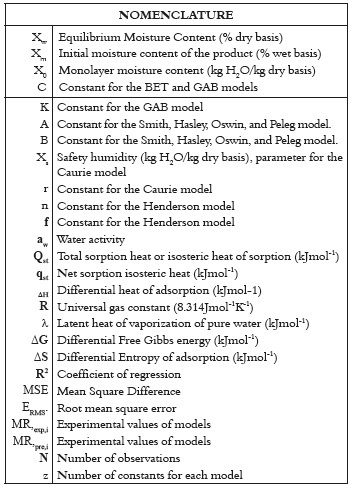
To obtain the sorption isotherms, the static gravimetric method was used (32 - 34). This method consists of using different saturated salt solutions to keep the relative humidity of the air in the containers at known and constant values. Table 1 shows the salts used to keep the product at a constant equilibrium relative humidity (water activity, aw) at temperatures of 4, 20, and 30°C. Three repetitions were performed for each water activity.
Panela samples weighing exactly 2.00 and 3.00 g were placed, in triplicate, in small glass containers. They were also placed in hermetically closed bottles before being taken to the climatic chamber (Dies CL 240). The samples were weighed periodically using scales (Boeco BBL-31) until equilibrium was reached. The equilibrium condition was established as the moment in which the difference between two consecutive weighings was not greater than 0.1% of the sample weight. The time required to reach equilibrium ranged from 4 to 6 weeks. The initial moisture content of the sample was determined by the Karl Fisher method. To inhibit microbial growth, a small container of salts of thymol, was placed in environments whose relative humidity was superior to 73%.
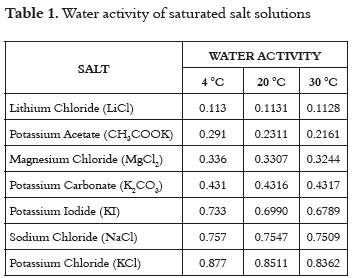
The experimental data regarding the sorption isotherms of the sugarcane powder were fitted to the BET, GAB, Caurie, Henderson, Smith, Hasley, Oswin, and Peleg mathematical models (13, 35 - 38). Table 2 shows the theoretical description of the models used in this study.
The parameters of the models were estimated using the non-linear regression procedure in the DATAFIT software, version 8.1.69 (Oakdale Engineering). The goodness of fit of each model for the experimental data was evaluated by determining the coefficient of determination (R2), the sum of the mean square difference (MSE), and the root mean square error ERMS. For a good fit, the values of R2 must be the greatest, and the MSE and ERMS values must be the lowest (39, 40).
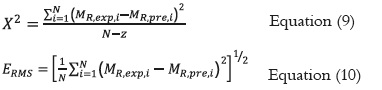
Where MR,exp,i, and MR,pre,i are the experimental and calculated values, N is the number of observations and z is the number of constants for each model.
Thermodynamic properties.
The net isosteric heat of sorption (qst) or differential heat of adsorption (ΔH) was obtained by adition total heat of sorption (Qst) from the vaporization enthalpy of water (λ). The Clausius- Clapeyron equation (Equation 11) was applied to the temperature-vapor pressure data of the product. The temperature intervals evaluated in the experiments permitted to assume a constant heat capacity value, thus, the enthalpy change is constant and non-variable (41 - 43).
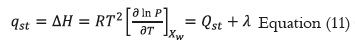
Where: is the pressure of water vapor in equilibrium with the material at an absolute temperature, Xw is the equilibrium moisture content in kg/kg dry solid and R is the universal gas constant.
The Gibbs free energy (ΔG) and entropy (ΔS) were calculated for the adsorption process by applying equation 12 and 13, respectively.
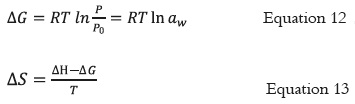
RESULTS
Characterization of Raw Material
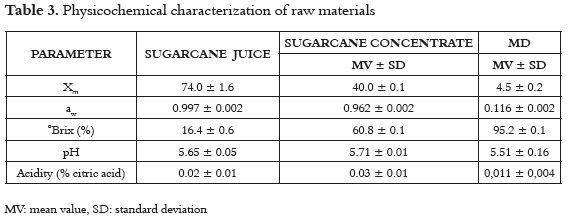
Table 3 shows the mean values and the standard deviation of the physicochemical properties of the sugarcane juice, sugarcane concentrate and maltodextrin. The ANOVA shows significant differences (p <0.05) to Xm, aw, ° Brix, pH and acidity, indicating that sugarcane concentrate presents greater stability (<aw), where the higher solids content and low moisture provide greater efficiency in the spray drying process (44).
Models for Adsorption Isotherms.
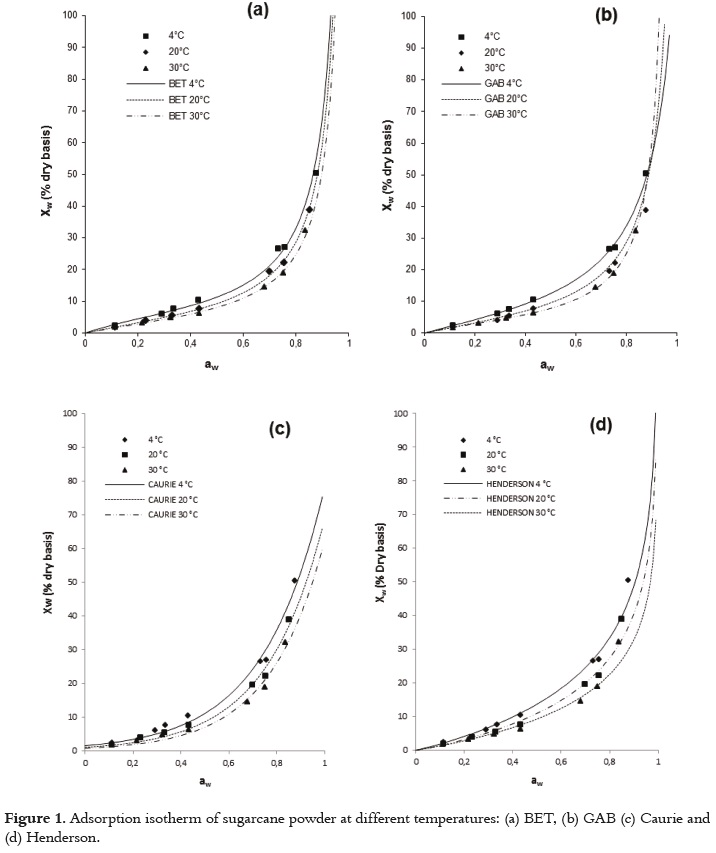
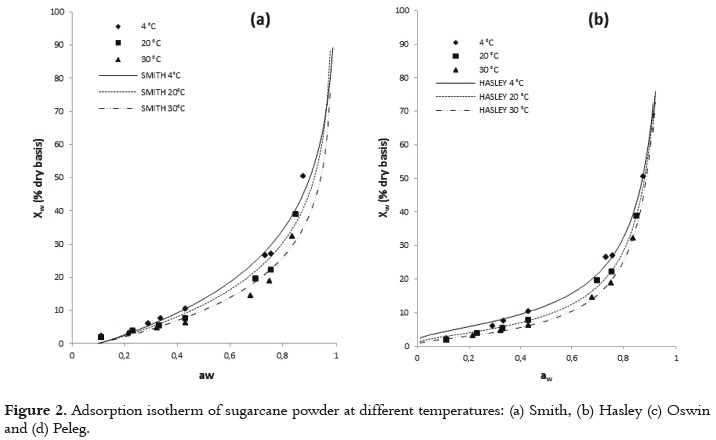
The usefulness of fit models is determined by their ability to predict new values when constants and other parameters obtained from experimental data are available (36). Figures 1 and 2 show the relationship between the water activity and equilibrium moisture content of sugarcane powder obtained by spray drying. At low and intermediate water activities, the so-called multilayer sorption region, moisture content increases linearly with aw (aw<0.6), whereas at high water activity levels, the so-called capillary condensation region, water content rapidly increases with aw (aw>0.6).
Table 4 shows the fitting models for the sugarcane powder obtained by spray drying. In general, all models tested show an acceptable goodness of fit (R2>0.97). The Table 4 shows that there exists a relation between the qualities of goodness of fit with the number of the model parameters. To the tested models, the GAB model has a higher number of unknown parameters (three unknown parameters) for which the non linear regression analysis showed the best goodness of fit. Similar results have been reported in the literature (45).
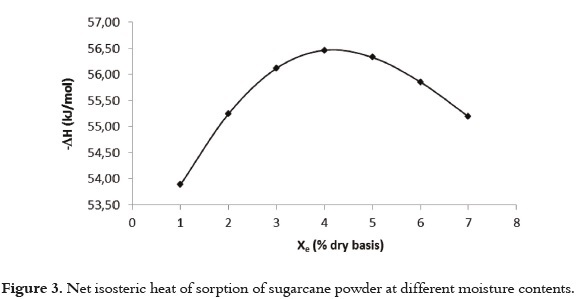
Table 5 shows the behavior of qst o r Δ H f or sugarcane powder using the GAB model and the Clausius Clayperon intervals based on temperature and moisture content. The results show a negative quantity, which illustrates the exothermic character of the process (adsorption) and the statistically significant interaction (P<0.05) with moisture content. Also, the moisture content dependence of net isosteric heat of sorption of water for sugarcane powder is shown in Figure 3.
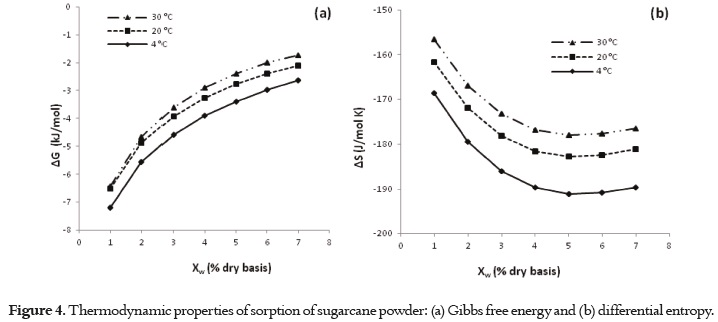
Figure 4 (a and b) shows the thermodynamic properties of sorption (ΔG and ΔS) obtained for sugarcane powder. The tendency showed by Gibbs free energy (Fig. 4a) explains that water absorption was a spontaneous phenomenon. In Fig. 4(b), the differential entropy (ΔS) is plotted as a function of moisture content where an increase in randomness is indicated by positive values for entropy change.
DISCUSSION
Sugarcane juice shows total soluble solids similar to those reported in panela production systems in Colombia (16-22%) (7). Additionally, the values found for pH and °Brix were to those reported (46) and higher than some varieties from India (47). Concentrated sugarcane juice shows higher solids content and lower moisture content than the initial product, because it was obtained before the last stage of the crystallization process (approximately > 82%) (47, 48). Maltodextrin (MD) is a low moisture powder with good solubility and no flavor or odor in the final product, and is considered to be a good encapsulate agent (49 - 51).
The adsorption isotherm of sugarcane powder has the type II BET classification shape for all studied temperatures, and not type III. This behavior could be explained by the presence of maltodextrin, which provides a high level of hygroscopicity to the mixture in relation to the sucrose from sugarcane juice at the same level of water activity. Several studies have reported a type II BET classification for maltodextrina (27, 28, 51). This sorption behavior in sugarcane powder has also been observed in products such as macaroni, mushrooms, onion powder, pineapple, powdered lactose, crystalline lactose, powdered and granular tea and oat biscuits (13, 52 - 59). Some studies, a type-III sigmoid curve has been observed in food that is rich in water-soluble components such as saccharose, fructose, glucose, corn syrup and lactose (17).
Figures 1 and 2 show that, when relative humidity is constant, moisture content decreases as the temperature increases. This is due to the higher vapor pressure at the food surface. This trend may be attributed to a reduction in the total number of active sites for water binding as a result of physical and/or chemical changes induced by temperature (14). Some authors consider that this phenomenon could be due to the fact that, after reaching higher energy contents, the structure undergoes physical and chemical changes that affect the weak chemical bonds between the active sites and water molecules (60). Additionally, the temperature increase creates an excitation at the molecular level, producing an increase in intermolecular distance and hence decreasing the molecular attraction forces of the water (12, 61). Other studies showed different behavior, where to give a specific level of water activity, the moisture content increased as temperature increased (52, 62).
Additionally, there is a relationship between the increase in temperature and the increase in water activity at constant moisture content, favoring microbial spoilage in food product. These behaviors have been described in other studies conducted on food matrices such as grape skin, tea, medlar, and quince (10, 63, 64). The increase in water activity after increasing the temperature is mainly due to changes in the fixation of water molecules on the solid matrix, dissociation of water molecules, or an increase in the solubility of solutes in the water. For higher water activity values, the soluble components of food have a larger amount of water available to solubilize. Thus the increase in temperature has a positive effect on this solubility.
The GAB model showed the higher degree of fit to the experimental data with high regression coefficients (R2) and minimal values of MSE and E(RMS) to all temperatures studied (Table 4). The values estimated with the Peleg and BET models also had a satisfactory fit for all the evaluated temperatures; however, there were small increases in the values of MSE and E(RMS). According to several authors, the GAB model has been used effectively for food whose water activity is below 0.4 (36, 42).
The moisture content of the monolayer (Xo) shows the amount of water that is strongly adsorbed on the food's surface and is considered as the optimal value to ensure the product's stability (14, 64, 65). For sugarcane powder, the GAB and BET models showed values from 4.7 to 9% at temperatures of 30 and 4 °C, respectively. It was observed that, in the GAB, BET and Caurie models, the moisture values for the monolayer (Xo) and the security humidity (Xs) decreased when an increase in temperature took place. This behavior has been observed in sugar-rich foods, in pineapple powder with an addition of 18% of maltodextrin and other foods (11, 12, 33, 38, 66, 67). A decrease in GAB monolayer moisture content (Xo) with increase in temperature was an indication that the absorbed molecules gained kinetic energy making the attractive forces to be loosened and this allowed some water molecules to break away from their sorption sites thus decreasing the equilibrium moisture values (67). Additionally, the monolayer capacity (Xo) in the BET model was less than that of the GAB model, while the energy constant C in the BET model was larger than that of the GAB model, where this behavior has been observed by several authors (36).
The heat required to remove water from sugarcane powder, starting with a moisture content of 0.08 kg/kg dry basis up to 0.01 kg/kg where the net isosteric heat of adsorption increased to a maximum value followed by a decrease, indicating that both adsorbate-adsorbent and adsorbate-adsorbate interactions were present. The initial increase in the net isosteric heat arose from the dominant adsorbate- adsorbate interactions, and the maximum value indicated the presence of an energetically heterogeneous surface (adsorbate-adsorbent interactions). The more negative the value of ΔH, the higher the degree of binding of water on the food surface (68).
The net isosteric heat increased to a maximum value (57 kJ mol-1) and then decreased with the increase in moisture content. The maximum net isosteric heat was obtained at moisture content of 4.7% (d.b.). This value is within the range of the monolayer moisture content found in the GAB and BET models. At monolayer moisture, the water is tightly bound to the material, corresponding with high interaction energy (69). A similar behavior was observed in another study conducted on apples (70). The increase in the net isosteric heat at low moisture contents can be explained by considering the exposure of sorption sites to higher amounts of binding energy that was not previously available. After the maximum, the decrease in the net isosteric heat with the amount of water sorbed can be qualitatively explained considering that initially, sorption occurs on the most active sites available, thus giving rise to greater interaction energy. As these sites become occupied, sorption occurs in fewer active sites, resulting in lower values for heat of adsorption. When the moisture content is very high, the adsorbate- adsorbent interactions are higher, decreasing the net isosteric heat of adsorption to values closer to the water's latent heat of condensation.
The results to ΔG shows a negative value, thus the adsorbed water is less free to escape into the atmosphere and an increase in temperature produces a decrease in adsorption capacity. Additionally, the changes in the free energy values indicated that sugarcane powder is very hygroscopic, showing that the free energy value increases when there is less water. Hence, the water molecules are caught more easily. A similar results has been reported in the literature (71).
The differential entropy showed strong dependence on the moisture content. The entropy change is higher at a temperature of 30ºC because the kinetic energy of the molecules interacting during the water exchange process is directly proportional to the temperature. Thus the entropy of the system is less at low temperatures due to the decrease in the number of sorption sites and restriction of movement of water molecules, thus rendering the adsorption process thermodynamically more favorable at lower temperatures.
The high values of ΔS at low moisture contents are explained by the fact that the water was tightly bound, but the lower entropy values can be interpreted as the water activity at which this product is most stable (53, 72, 73). The subsequent decrease in ΔS reflected the presence of more freely held water molecules and the formation of multi-layers (59). Similar results have been reported to medlar, yucca, melon seeds, soy, pineapple and orange tree leaves (10, 56, 61, 74, 75).
CONCLUSIONS
According to the results of this research project, the sorption isotherm for sugarcane and maltodextrin powder obtained by spray drying corresponds to type II. The GAB equation showed a better fit to the experimental data modeling (0.11≤aw≤0.87), although all models showed validity and goodness of fit to the experimental data.
The effect of the temperature on the moisture sorption isotherms evidences that the sugarcane powder obtained through spray drying using maltodextrin as additive can show hygroscopic characteristics as temperature increases. The molecular system of sugarcane powder obtained through spray drying using maltodextrin as an additive can be very unstable, since very little energy is required to destabilize it; this can easily generate hydration or dehydration processes. It is recommended that a study of phase transitions in the dust of sugarcane to supplement their evaluation of adsorption thermodynamics.
Conflict of interest
The authors declare that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Limitations
Due to the high-energy interaction between sugarcane powder and its environment, it is recommended to conduct a study of phase transitions to complement the evaluation of adsorption thermodynamics.
REFERENCES
1. Sampietro D, Vattuone MA, Isla MI. Plant growth inhibitors isolated from sugarcane (Saccharum officinarum) straw. J Plant Physiol. 2006 Julio 3; 163 (8): 837-846. [ Links ]
2. Rodriguez G., Garcia H., Diaz Z., Santacoloma P. Producción de Panela como estrategia de diversificación en la generación de ingresos en áreas rurales de América Latina. AGSF (Servicio de Gestión, Comercialización y Finanzas Agrícolas). Roma, Italia: FAO. 2004. 81 p. [ Links ]
3. Ministerio de Agricultura y Desarrollo Rural Observatorio Agrocadenas Colombia. Características y estructura de la cadena agroindustrial de la panela en Colombia. Documento de trabajo Número 12. Bogotá, Colombia: MADR, Ministerio de Agricultura y Desarrollo Rural. 2004. 12 p. [ Links ]
4. Castellanos OF, Torres LM, Flórez DH. Agenda prospectiva de investigación y desarrollo tecnológico para la cadena productiva de la panela y su agroindustria en Colombia. Bogotá, Colombia: Giro Editores Ltda. 2009. 258 p. [ Links ]
5. Rodríguez E. La Agrocadena panelera y el alcohol carburante en Boyacá. Cenes. 2004; 24 (37): 157 - 173. [ Links ]
6. Ministerio de Agricultura y Desarrollo Rural Observatorio Agrocadenas Colombia. La cadena de la panela en Colombia. Una mirada global de su estructura y dinámica 1991-2005. Documento de trabajo número 103. Bogotá, Colombia: Ministerio de Agricultura y Desarrollo Rural. 2006. 24 p. [ Links ]
7. Osorio G. Buenas Prácticas Agrícolas -BPA- y Buenas Prácticas de Manufactura -BPM- en la Producción de Caña y Panela. Medellín, Colombia: Corpoica, Mana, FAO. 2007. 200 p. [ Links ]
8. Gabas AL, Telis-Romero J, Giraldo-Gómez GI, Nicoletti-Telis VR. Propiedades termodinámicas de sorción de agua de la pulpa de lulo en polvo con encapsulantes. Ciênc Tecnol Aliment. 2009 Oct-Dec; 29 (4): 911-918. [ Links ]
9. Al-Muhtaseb AH, Mcminn WAM, Magee TRA. Moisture sorption isotherm characteristics of food products: a review. Food Bioprod Process. 2002 Jun; 80 (2): 118-128. [ Links ]
10. Moreira R, Chenlo F, Torres M.D, Vallejo N. Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. J Food Eng. 2008 Oct; 88 (4): 514-521. [ Links ]
11. Moraga G, Igual M, García E, Mosquera LH, Martínez N. Effect of relative humidity and storage time on the bioactive compounds and functional properties of grape fruit powder. J Food Eng. 2012 Oct; 112 (3): 191-199. [ Links ]
12. Ayala A. Estimación de las isotermas de adsorción y del calor isostérico en harina de yuca. Rev Bio Agro. 2012 Jan-Jun; 9 (1): 88 - 96. [ Links ]
13. Sinija VR, Mishra HN. Moisture sorption isotherms and heat of sorption of instant (soluble) green tea powder and green tea granules. J Food Eng. 2008 Jun; 86 (4): 494-500. [ Links ]
14. Goula AM, Karapantsios TD, Achilias DS, Adamopoulos KG. Water sorption isotherms and glass transition temperature of spray dried tomato pulp. J Food Eng. 2008 Mar; 85 (1): 73-83. [ Links ]
15. Chen C. Sorption isotherms of sweet potato slices. Biosystems Eng. 2002 Sep; 83 (1): 85-95. [ Links ]
16. Chen, C. Moisture sorption isotherms of pea seeds. J Food Eng. 2003 Jun; 58 (1): 45-51. [ Links ]
17. Martínez N, Andrés AM, Chiralt A, Fito P. Termodinámica y cinética de sistemas alimento entorno. Valencia, España: Universidad Politécnica de Valencia. Servicio de publicaciones. 1998. 372 p. [ Links ]
18. McMinn WAM, Magee TRA. Thermodynamics properties of moisture sorption of potato. J Food Eng. 2003 Nov; 60 (2): 157-165. [ Links ]
19. Telis VRN, Gabas AL, Menegalli FC, Telis J. Water sorption thermodynamic properties applied to persimmon skin and pulp. Thermochim Acta. 2000 Jan; 343 (1-2): 49-56. [ Links ]
20. Karatas S, Esin A. A laboratory scraped surface drying tomato paste. Lebens Wissen Technol. 1990; 23 (4): 354-357. [ Links ]
21. Bhandari BR, Senoussi A, Dumoulin ED, Lebert A. Spray drying of concentrated fruit juices. Dry Technol. 1993; 11 (5): 1081-1092. [ Links ]
22. Dolinsky A, Maletskaya K, Snezhkin Y. Fruit and vegetable powders production on the bases of spray and convective drying methods. Dry technol. 2000; 18 (3); 747-758. [ Links ]
23. Truong V, Bhandari B, Howes T. Optimizations of concurrent spray drying process for sugar-rich foods. Part II- optimization of spray drying process based on glass transition concept. J Food Eng. 2005 Nov; 71 (1): 66-72. [ Links ]
24. Jayasundera M, Adhikari B, Adhikari R, Aldred P. The effects of proteins and low molecular weight surfactans on spray drying of model sugar-rich foods: powder production and characterization. J Food Eng. 2011 May; 104 (2): 259-271. [ Links ]
25. Vega C, Roos YH. Invited Review: Spray dried, dairy and dairy like emulsions, composition considerations. J Dairy Sci. 2006 Feb; 89 (2): 383-401. [ Links ]
26. Gaiani C, Morand M, Sanchez C, Arab M, Jacquot P, Schuck R, Jeantet E, Scher J. How surface composition of high milk protein powders is influenced by spray drying temperature. Colloid Surface B. 2010 Jan; 75 (1): 377-384. [ Links ]
27. Roos YH. Phase Transitions in Foods. San Diego, CA, USA. Academic Press. 1995. 360p. [ Links ]
28. Bhandari BR, Datta N, Howes T. Problems associated with spray drying of sugar rich foods. Dry Technol. 1997 (15); 671-684. [ Links ]
29. Poaquiza D. Determinación de isotermas y calor de sorción de humedad de panela granulada producida por las organizaciones paneleras de Ingapi y Pacto. [Tesis pregrado]. [Quito, Ecuador]: Escuela Politécnica Nacional; 2008. 145p. [ Links ]
30. Horwitz W (editor). Official Methods of Analysis of AOAC International. 18th edition. Virginia, USA: AOAC International; 2005, Chapter 37. Fruits and fruit products; p. 1. [ Links ]
31. Largo E. Desarrollo de un producto en polvo a partir del jugo de caña panelera utilizando secado por aspersión. [Tesis de Maestría]. [Medellín, Colombia]: Universidad Nacional de Colombia - Medellín; 2012. 66 p. [ Links ]
32. Cruz S, Zapata C, Ferrereira L, Gomes A, Olivera F. Adsorption isotherms of pinhão (Araucaria angustifolia seeds) starch and thermodynamic analysis. J Food Eng. 2010 Oct;100 (3): 468-473. [ Links ]
33. Gabas AL, Telis V, Sobral PJ, Telis-Romero J. Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. J Food Eng. 2007 Sep; 82 (2): 246-252. [ Links ]
34. Cassini AS, Marczak LD, Noreña CP. Water adsorption isotherms of texturized soy protein. J Food Eng. 2006 Nov; 77 (1): 194-199. [ Links ]
35. Furmaniak S, Terzyk AP, Gaudena PA. The general mechanism of water sorption on foodstuffs - Importance of the multi temperature fitting of data and the hierarchy of models. J Food Eng. 2007 Oct; 82 (4): 528-535. [ Links ]
36. Timmermann EO, Chirife J, Iglesias HA. Water sorption isotherms of food and foodstuffs: BET or GAB parameters?. J Food Eng. 2001 Apr; 48 (1): 19-31. [ Links ]
37. Perdomo J, Cova A, Sandoval AJ, García L, Laredo E, Müller AJ. Glass transition temperatures and water sorption isotherms of cassava starch. Carbohyd Polym. 2009 Mar; 76 (2): 305-313. [ Links ]
38. Djendoubi NM, Bonazzi C, Boudhrioua N, Kechaoub N, Courtois F. Influence of sugar composition on water sorption isotherms and on glass transition in apricots. J Food Eng. 2012 Jul; 111 (2): 403-411. [ Links ]
39. Demir V, Gunhan T, Yagcioglu AK, Degirmencioglu A. Mathematical modelling and the determination of some quality parameters of air-dried bay leaves. Biosystems Eng. 2004 Jul; 88 (3): 325-335. [ Links ]
40. Goyal RK, Kingsly ARP, Manikantan MR, Ilyas SM. Thin-layer Drying Kinetics of Raw Mango Slices. Biosystems Eng. 2006 Sep; 95 (1): 43-49. [ Links ]
41. Yan Z, Sousa-Gallagher MJ, Oliveira F. Sorption isotherms and moisture sorption hysteresis of intermediate moisture content banana. J Food Eng. 2008 Jun; 86 (3): 342-348. [ Links ]
42. Bell LN, Labuza TP. Moisture Sorption: Partial aspects of isotherms measurement and use. St. Paoul, Minnesota, USA: Amer Assn of Cereal Chemists; 2000. 122 p. [ Links ]
43. Myers AL. Thermodynamics of adsorption in porous materials. AiChE J. 2002 Jan; 48 (1): 145-160. [ Links ]
44. Masters K. Spray drying handbook. Burnt Mill, Harlow, Essex, England: Longman Scientific and Technical; 1991. 725 p. [ Links ]
45. Furmaniak S, Terzyk AP, Gotembiewski R, Gauden PA, Czepirski L. Searching the most optimal model of water sorption on foodstuffs in the whole range of relative humidity. Food Res Int. 2009 Oct; 42 (8): 1203-1214. [ Links ]
46. Yusof S, Shian LS, Osman A. Changes in quality of sugar-cane juice upon delayed extraction and storage. Food Chem. 2000 Mar; 68 (4): 395 - 401. [ Links ]
47. Jagannadha PVK, Das M, Das SK. Changes in physical and thermo-physical properties of sugarcane, palmyra-palm and date-palm juices at different concentration of sugar. J Food Eng. 2009 Feb; 90 (4): 559-566. [ Links ]
48. Guzmán G, Castaño J. Secado por atomización del jugo de la caña de azúcar. Cenicafé. 2002 Nov; 53 (4): 327-333. [ Links ]
49. Kenyon MM. Modified starch, maltodextrin, and corn syrup solids as wall materials for food encapsulation. Acs Sym Ser. 1995 Mar 24; 590: 42-50. [ Links ]
50. Wang YJ, Wang L. Structures and properties of commercial maltodextrins from corn, Potato and rice starches. Starch. 2000 Sep; 52 (8-9): 296-304. [ Links ]
51. Takeiti CY, Kieckbuschb TG, Collares-Queiroz FP. Optimization of the jet steam instantizing process of commercial maltodextrins powders. J Food Eng. 2008 Jun; 86: 444-452 [ Links ]
52. Arslan N, Togrul H. Modelling of water sorption isotherms of macaroni stored in a chamber under controlled humidity and thermodynamic approach. J Food Eng. 2005 Jul; 69 (2): 133-145. [ Links ]
53. Pérez C, Beristain CI, Lobato C, Rodríguez ME, Vernon EJ. Thermodynamic analysis of the sorption isotherms of pure and blended carbohydrate polymers. J Food Eng. 2006 Dec; 77 (4): 753-760. [ Links ]
54. Shivhare U, Arora S, Ahmed J, Raghavan G. Moisture adsorption isotherms for mushroom. LWT- Food Sci Technol. 2004 Feb; 37 (1): 133-137. [ Links ]
55. Sukumar D, Hemavathy J, Bhat K. Moisture sorption studies on onion powder. Food Chem. 2002 Sep; 78 (4): 479-482. [ Links ]
56. Simal S, Femenia A, Palou A, Rossello C. Water desorption thermodynamic properties of pineapple. J Food Eng. 2007 Jun; 80 (4): 1293-1301. [ Links ]
57. Bronlund J, Paterson T. Moisture sorption isotherms for crystalline, amorphous and predominantly crystalline lactose powders. Int Dairy J. 2004 Mar; 14 (3): 247-254. [ Links ]
58. Mathlouthi M, Rogé B. Water vapor sorption isotherms and the caking of food powders. Food Chem. 2003 Jul; 82 (1): 61-71. [ Links ]
59. McMinn WAM, McKeea DJ, Mageea TR. Moisture adsorption behavior of oatmeal biscuit and oat flakes. J Food Eng. 2007 Mar; 79 (2): 481-493. [ Links ]
60. Mazza G. Thermodynamic considerations of water vapour sorption by horseradish roots. Lebensm Wiss Technol. 1980; 13: 13-17. [ Links ]
61. Mohamed A, Kouhila M, Jamali A, Lahsasni S, Mahrouz M. Moisture sorption isotherms and heat of sorption of bitter orange leaves (Citrus aurantium). J Food Eng. 2005 Apr; 67 (4): 491-498. [ Links ]
62. Das M, Das SK. Analysis of moisture sorption characteristics of fish protein myosin. Int J Food Sci Technol. 2002 Feb; 37 (2): 223-227. [ Links ]
63. Kaya S, Kahyaoglu T. Thermodynamic properties and sorption equilibrium of pestil (grape leather). J Food Eng. 2005 Nov; 71 (2): 200-207. [ Links ]
64. Arslan N, Togrul H. The fitting of various models to water sorption isotherms of tea stored in a chamber under controlled temperature and humidity. J Stored Prod Res. 2006; 42 (2): 112-135. [ Links ]
65. Andrade RD, Lemus MR, Pérez CE. Models of sorption isotherms for food: uses and limitations. Vitae. 2011; 18 (3): 325-334. [ Links ]
66. Argyropoulos D, Alex R, Kohler R, Müller J. Moisture sorption isotherms and isosteric heat of sorption of leaves and stems of lemon balm (Melissa officinalis L.) established by dynamic vapor sorption. LWT - Food Sci Technol. 2012 Jul; 47 (2): 324-331. [ Links ]
67. Oluwamukomi MO. Adsorption isotherm modeling of soymelon- enriched and un-enriched 'Gary' using GAB equation. Afr J Food Sci. 2009 May; 3 (5): 117-124. [ Links ]
68. Falade KO, Aworh OC. Adsorption isotherms of Osmo-oven dried African start apple (Chrysophyllum albidum) and African mango (Irvingia gabonensis) slices. Eur Food Res Technol. 2004 Feb; 218 (3): 278-283. [ Links ]
69. Quirijns EJ, Van Boxtel AJ, Van Loon WK, Van Straten G. Sorption isotherms, GAB parameters and isosteric heat of sorption. J Sci Food Agr. 2005 Aug; 85 (11): 1805-1814. [ Links ]
70. Moraes MA, Rosa GS, Pinto LA. Moisture sorption isotherms and thermodynamic properties of apple Fuji and garlic. Int J Food Sci Technol. 2008 Oct; 43 (10): 1824-1831. [ Links ]
71. Vigano J, Azuara E, Telis VR, Beristain CI, Jiménez M, Telis J. Role of enthalpy and entropy in moisture sorption behavior of pineapple pulp powder produced by different drying methods. Thermochim Acta. 2012 Jan 20; 528: 63-71. [ Links ]
72. Rizvi SS, Benado AL. Thermodynamics properties of dehydrated food. Food Technol. 1984 Mar; 38 (3): 83-92. [ Links ]
73. Domínguez IL, Azuara E, Vernon-Carter EJ, Beristain CI. Thermodynamic analysis of the effect of water activity on the stability of macadamia nut. J Food Eng. 2007 Aug; 81 (3): 566-571. [ Links ]
74. Aviara NA, Ajibola OO. Thermodynamics of moisture sorption in melon seed and cassava. J Food Eng. 2002 Nov; 55 (2): 107-113. [ Links ]
75. Aviara NA, Ajibola OO, Oni SA. Sorption equilibrium and thermodynamic characteristics of soya bean. Biosyst Eng. 2004 Feb; 87 (2): 179-790. [ Links ]