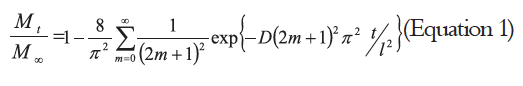Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Vitae
versão impressa ISSN 0121-4004
Vitae vol.22 no.2 Medellín maio/ago. 2015
https://doi.org/10.17533/udea.vitae.v22n2a05
DOI:10.17533/udea.vitae.v22n2a05
FOODS: SCIENCE, ENGINEERING AND TECHNOLOGY
FABRICATION OF AN ANTIMICROBIAL ACTIVE PACKAGING AND ITS EFFECT ON THE GROWTH OF Pseudomonas AND AEROBIC MESOPHILIC BACTERIA IN CHICKEN
ELABORACIÓN DE UN ENVASE ACTIVO ANTIMICROBIANO Y SU EFECTO EN EL DESARROLLO DE Pseudomonas Y BACTERIAS AEROBIAS EN POLLO
Odilia Azucena HIGUERA-BARRAZA BEng.1, Herlinda SOTO-VALDEZ PhD.1, Evelia ACEDO-FÉLIX PhD.1†, Elizabeth PERALTA M.Sc.1*
1 Centro de Investigación en Alimentación y Desarrollo, A.C. Apdo. Postal 1735. Hermosillo, Sonora 83304, México.
†Deceased.
* Corresponding author: peralta@ciad.mx.
Recibido: Marzo 31 de 2015
Aceptado: Septiembre 21 de 2015
ABSTRACT
Background: One of the aims of food packaging is to protect the product from environmental factors that can cause a reduction in quality. Surface growth of microorganism is one of the leading causes of food spoilage. One option is to use antimicrobial packaging to provide an increased margin of safety and quality. Objectives: The aim of this study was evaluate the effect of active packaging with eugenol on growth of Pseudomonas and aerobic mesophilic bacteria in fresh chicken pieces. Methods: Three batches of low-density polyethylene (LDPE) film, containing 0, 9.0 and 7.7, mg g-1 eugenol (control, AAF1 and AAF2, respectively), were extruded in a pilot-plant scale blown-extrusion machine. The films with eugenol lost 42.7% and 36.8% (AAF1 and AAF2, respectively) of eugenol during processing and absorbed UV-visible light at 300-261 nm. The kinetics of eugenol release from the AAF1 into the air at 5°C and 25°C displayed Fick's behavior, and a diffusion coefficient of 10-8 cm2 s-1 was calculated. Results: Eugenol showed antimicrobial activity on in vitro, using paper discs with 1.74, 0.87 and 0.36 mg eugenol on 108 CFU mL-1 of Pseudomonas fluorescens in Muller-Hinton agar. Chicken thighs were wrapped in the AAF2 film, and the effects on the growth of Pseudomonas and aerobic mesophilic bacteria (AMB) were evaluated after storage for 5 d at 5°C. The AAF2 showed a moderately antimicrobial effect in reducing the growth of Pseudomonas (1.1 x 106 CFU g-1) relative to growth in the control film (6.0 x 106 CFU g-1) (P < 0.05). The film with eugenol was effective in reducing the growth of AMB (9.0 x 105 CFU g-1) relative to growth in the control film (1.7 x 106 CFU g-1) (P < 0.05). Conclusions: Despite the high losses of eugenol during the extrusion of the films, they showed an antimicrobial effect during contact with fresh chicken under commercial conditions. This study shows the potential use of eugenol for application in LDPE antimicrobial packaging film.
Keywords: Antimicrobial active packaging, diffusion of eugenol, Pseudomonas fluorescens.
RESUMEN
Antecedentes: Uno de los principales objetivos del envasado de alimentos es protegerlo de factores que puedan afectar y causar una reducción en la calidad. El desarrollo de microorganismos en la superficie es uno de las causas principales del deterioro de los alimentos. Una opción es el empleo de envases con propiedades antimicrobianas. Objetivos: El objetivo del presente estudio fue evaluar el efecto de un envase antimicrobiano conteniendo eugenol en el desarrollo de Pseudomonas y bacterias mesofílicas aerobias (BMA) en piezas de pollo. Métodos: Tres lotes de película de polietileno de baja densidad (PEBD) conteniendo 0, 9.0 y 7.7 mg g-1 de eugenol (control, AAF1, AAF2, respectivamente) fueron obtenidas por extrusión-soplo utilizando un extrusor a nivel planta piloto. Se calculó la cinética de liberación del eugenol de la AAF1 hacia el aire a 5°C y 25°C. Se evaluó la capacidad antimicrobiana in vitro del eugenol sobre 108 UFC mL-1 de Pseudomona fluorescens utilizando discos de papel conteniendo 1.74, 0.87 y 0.36 mg de eugenol en agar Muller-Hinton. Las piezas de pollo fueron envueltas en la película AAF2 y almacenadas a 5°C evaluando a los 5 días el efecto de la película en el desarrollo de Pseudomonas y en BMA. Resultados: El eugenol mostró actividad antimicrobiana inhibiendo el crecimiento de P. fluorescens. Las películas conteniendo eugenol perdieron durante el proceso de extrusión 42.7% y 36.8% (AAF1 y AAF2 respectivamente) del total añadido mostrando un comportamiento fickiano con un coeficiente de difusión de 10-8 cm2 s-1. Las AAF2 mostraron un efecto moderado en la reducción del desarrollo de Pseudomonas (1.1 x 106 CFU g-1) comparadas con el control (6.0 x 106 CFU g-1) (P < 0.05). Las películas con eugenol (AAF2) fueron efectivas al reducir el desarrollo de las BMA (9.0 x 105 CFU g-1) comparadas con la película control (1.7 x 106 CFU g-1) (P < 0.05). Conclusiones: A pesar de las pérdidas del eugenol durante el proceso de extrusión para la obtención de las películas, estas mostraron un efecto antimicrobiano sobre las piezas de pollo. Por lo tanto, este estudio muestra el uso potencial del eugenol para la aplicación en envases antimicrobianos elaborados a base de PEBD.
Palabras clave: Envases activos antimicrobianos, coeficiente de difusión, eugenol, Pseudomonas fluorescens.
INTRODUCTION
In recent years, chicken consumption has maintained a steady growth trend, representing over 43% of meat consumption in Mexico [1]. Global consumption of chicken in American continent is almost three times the global average. In the EU consumption of meat per capita, which reached its lowest level in 2013 in the last 11 years (64.7 kg), it is expected to recover from this year. Thus, in 2023 it is expected that consumption per capita will reach 66.1 kg and projecting a global growth by about 2.8% annually from 2013 to 2022; this growth trend is mainly influenced by an increased demand for white meat, which is low in fat [2-4]. Fresh chicken is commonly consumed due to its nutritional profile, versatility and low price. The increased demand for fresh poultry and the need for transportation to distant markets have increased the necessity for extending the shelf life of poultry products [5].
Chicken is a highly perishable product even when stored under chilled conditions. Chicken products have a high number of spoilage microorganisms and may contain pathogenic bacteria. The normal refrigerated shelf life of fresh chicken is less than 5 days from the time of slaughter [6]. Bacteria from the genus Pseudomonas are considered the major producers of the volatile compounds responsible for altered flavors (aldehydes, ketones and esters) in foods. Pseudomonas fluorescens (P. fluorescens) is one of the most predominantly isolated bacteria associated with the spoilage of fresh poultry [7].
One of the aims of food packaging is to protect the product from environmental factors that can cause a reduction in quality [8]. Efforts have been made to minimize food-package interactions, such as migration, sorption and gas permeability [9]. However, these interactions have also been used in a positive way to improve the protection capacity of the package, such as in active packaging systems. Active packaging can be defined as a type of container that includes additives that help to extend the shelf life of the food by preserving the quality longer than conventional packaging [10]. These materials provide additional functions that in some way enable the package to interact with the food to improve its quality, safety and convenience. The release of volatile compounds from packaging materials is a mass transfer process by which low molecular mass substances initially present in the package are released into the contained product.
The diffusion coefficient (D) measures the rate at which the release process occurs, and it is described by Fick's second law [11].
Surface growth of microorganisms is one of the leading causes of food spoilage [12] directly affecting the quality and safety of the food. One option for decreasing the surface spoilage of foods is to incorporate antimicrobial agents into the packaging material to provide an increased margin of safety and quality. Antimicrobial food packaging acts to reduce, inhibit or retard the growth of microorganisms that may be present in the packaged food [13]. A packaging system that allows for the slow release of an antimicrobial agent into the food could significantly increase the shelf life and retain the quality of a variety of foods [14]. Several articles have reported the use of antimicrobial agents to formulate antimicrobial packaging [6, 13–18]. Essential oils and their components have antimicrobial properties against microorganisms (including Gram-positive and Gram-negative bacteria) [13, 14, 16, 19–24]. The use of essential oils (e.g., cinnamon, oregano, clove, eucalyptus, lavender, lemongrass, lemon, lime, orange, peppermint, basil, wintergreen and thyme) as antimicrobial agents, in vitro and in foodstuffs, has been reported in several studies [13, 16, 20–26]. The FDA has categorized clove oil as generally recognized as safe (GRAS) for use in dental cement and as a food additive (CFR 184.1257). Eugenol (4-allyl-2-methoxyphenol) is a naturally occurring phenolic compound that is the major essential oil component of cloves (90.1%). Eugenol shows absorption of UV light with a maximum absorption at 265 nm. It is a volatile liquid at room temperature, with moderate water solubility (2.46 mg L-1 at 25°C). It has shown antioxidant and antimicrobial properties [27]. It has been reported that clove oil and eugenol can affect P. fluorescens growth [28]. Eugenol has shown great potential for use as an active additive in food packaging applications. The diffusion of pure eugenol into plastic films has been previously tested [29], but its release as an additive from packaging has not been evaluated.
When designing antimicrobial active packaging, it is important to consider the effects of the package fabrication process on the active additive. In the case of chicken packaging, the effects of the film extrusion process must be determined. The aim of this work was to manufacture antimicrobial active films (AAFs) using an extrusion-blow process, with eugenol incorporated into the polymeric matrix. An AAF1 was manufactured to measure the kinetics of the release of eugenol at 5°C and 25°C (AAF1). Then, another batch was manufactured under the same conditions as above (AAF2) to determine the effectiveness of the film on fresh chicken pieces (thighs) by following the growth of Pseudomonas spp. and aerobic mesophilic bacteria (AMB) during storage at 5°C.
MATERIALS AND METHODS
Materials
Low-density polyethylene LDPE (Certene) was obtained from Muelhstein (Houston, TX, USA) with a 110°C of melting point, 2.3 g/10 min of melt index and 0.924 g cm-3 of density. Eugenol was purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade water and methanol were supplied by JT Baker (Ecatepec, México). Fresh chicken thighs were purchased from a local supermarket in Hermosillo, Mexico.
Organisms and media
P. fluorescens of American Type culture collection (ATCC) 13525 (Manassas, VA, USA) was kept at 5°C in nutrient broth and agar. Mueller-Hinton Agar, Pseudomonas Agar F and Plate Count Agar were obtained from Difco (Becton Dickinson, Franklin Lakes, NJ, USA). NaCl, phosphate buffer (monobasic and dibasic sodium phosphate), Tween 80 and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Film manufacturing
The AAFs were produced on a pilot-plant scale extruder. Two batches of pellets containing the antimicrobial agent were produced by mixing LDPE with 9.0 and 7.7 mg g-1 of eugenol (AAF1 and AAF2, respectively). A pilot-plant scale extrusion machine with three heating zones at 130°C, equipped with a filament die and coupled to a pelletizer (Beutlespacher, Mexico, D.F.), was used. Films were obtained from the two formulations by using the blow extrusion process with a pilot-plant sized mono-spindle extruder with four heating zones at 130°C (Beutelspacher, Mexico D.F., México) at the Centro de Investigación en Alimentación y Desarrollo, A.C. campus Hermosillo, Mexico. A control film without eugenol was also prepared under the same conditions. The thickness of the films was measured with a model DTT micrometer from E.J. Cady & Co. (Wheeling, IL, USA). Ten film specimens were measured, and data were expressed in micrometers (µm). The AAF1 was produced to determine the kinetics of release, immediately after obtaining the film. The AAF2 was produced one month later under the same conditions than AAF1. This film was stored at -20°C until the in vitro antimicrobial effect and packaging of chicken pieces were performed.
Extraction and quantification of eugenol in the films
AAF1, AAF2 and control films were extracted using methanol with constant stirring at 40°C for 24 h. The extraction was repeated to ensure complete extraction of eugenol. The extracts were filtered into amber vials with 0.22 µm membrane Durapore® filters (Millipore Corporation, Bedford, MA, USA). The eugenol quantification was performed by reverse-phase high-performance liquid chromatography (HPLC) using a liquid chromatograph (Varian 9012, Mexico) coupled to a fluorescence detector (Varian 9075, Mexico) at an excitation wavelength of 272 nm and an emission wavelength of 298 nm. The liquid chromatograph was equipped with a 150 x 4.6 mm C18 ChromSep column (Varian, Mexico) protected with a C18 guard column (50 mm). A 10 µL sample volume was injected into the HPLC using a Rheodyne 7125 injector (Rheodyne, Bershire, UK) and was eluted (isocratic flow) with 85:15 methanol:water at 1 mL min-1. Three replicates were performed for each sample. A calibration curve for eugenol was prepared by making solutions ranging from 0.5 to 9.0 μg mL-1 of eugenol in methanol. The retention time for eugenol was 2.6 min, and the limit of quantification in methanol (LOQ) was 0.012 µg mL-1.
Release of eugenol from the AAF1 at 5°C and 25°C
Fifty-four film samples of AAF1 (2.5 x 5 cm) were exposed to the environment (both sides). Thirty pieces were exposed at 5°C (recommended temperature for storing fresh chicken), and twenty-four pieces were exposed at 25°C (room temperature). The release of eugenol was monitored periodically by taking sets of three pieces of film during the incubation periods of 20 h at 5°C and 8 h at 25°C. Eugenol was quantified in the films, as described in the previous section.
The release process can be described as the kinetics of the diffusion of the volatile compound in the film, and it is expressed as D (diffusion coefficient). An analytical solution for the equation describing Fick's second law, which describes diffusion in two dimensions and at an infinite volume (environment) [30, 31], was used to determine the value of D.
where Mt /M∞ is the concentration of eugenol released at time t divided by the concentration of eugenol released at equilibrium; and l is the thickness of the film (cm). D is the diffusion coefficient (cm2 s-1), which was calculated using Eq. (1) by plotting the ratio of t vs. Mt /M∞. D was determined by minimizing the sum of the squares of errors (SSE) between the measured and estimated values. The MATLAB program was used to find the best fitting of the data to Eq. (1) by using the non-linear regression (nlinfit) function in MATLAB R2008b (MathWorks, Natick, MA, USA) [32].
In vitro antibacterial activity of eugenol on P. fluorescens
The agar diffusion method was used to determine the antibacterial activity of eugenol. Sterile paper discs (5 mm diameter) containing eugenol were prepared for the in vitro antibacterial activity test in accordance with the method of [33]. Overall, 10 µL of different eugenol solutions, which were prepared at dilutions of 1:1, 1:5 and 1:10 with Tween 80 (10%) and DMSO (0.5%), were loaded on sterile paper discs. Sterile discs with phosphate buffer and Tween 80 in DMSO (0.5%) were used as negative controls.
The discs were set on the surface of Muller-Hinton agar dishes freshly loaded with 108 CFU mL-1 of P. fluorescens, and the dishes were incubated at 25°C for 24 h. The analysis was carried out using three discs for each dilution, and the diameters of inhibition zones around the discs were measured (mm).
The minimal inhibitory concentration (MIC) of eugenol was determined by the agar diffusion method according the previously described technique. The minimum concentration showing a clear zone of 10 mm or less was taken as the MIC.
The ANOVA test was performed for each eugenol dilutions disc, and significant differences between the mean diameters of inhibition zones around the discs were determined by the Tukey-Kramer test (P < 0.05) using Number Cruncher Statistical Systems (NCSS) [34].
Antimicrobial effect of AAF2 on fresh chicken
Four pouches (17 x 8 cm) made of the AAF2 and control films were used for wrapping fresh chicken thighs under hygienic conditions. All treatments were stored for 5 days at 5°C. Samples (duplicates) of all treatments were taken at 0 and 5 days of storage to evaluate Pseudomonas and AMB survival. The counting of Pseudomonas and AMB was performed in accordance with the procedure established by NOM-092-SSA1-1994 [35], and NOM-110-SSA1-1994 [36]. For Pseudomonas counts, serial dilution of the samples was performed in buffered phosphate, and 0.1 mL of each dilution was spread on Pseudomonas Agar F. Incubation was carried out at 26 ± 1°C for 24-48 h, and values were reported as CFU g-1.
The ANOVA test was performed, and significant differences between the means of the counts obtained from the antimicrobial active packaging and the control packaging were determined by the Tukey-Kramer test (P < 0.05) using NSCC [34].
RESULTS
Thickness of the AAF1 with eugenol
The thicknesses of the AAF1, AAF2 and control films were 27.9 ± 1.85, 26.4 ± 2.79 and 20.8 ± 3.93 µm, respectively. Differences in the thicknesses values of films prepared due to variations in the output (gap) of the extruder die. There were no significant differences in the values of the film elaborated.
The effect of processing conditions on the concentration of eugenol in the AAF and on eugenol release
The concentrations of eugenol in the AAF1 and AAF2 were 5.16 ± 0.06 mg g-1 and 4.87 ± 1.02 mg g-1, respectively. These values are 57.3% and 63.2% of the eugenol originally added before processing respectively.
Release of eugenol from the AAF1 at 5°C and 25°C
Figure 1 shows the release of eugenol in the AAF1 at different temperatures. At 5°C, the concentration of eugenol decreased from 5.16 ± 0.78 mg g-1 to 0.28 ± 0.04 mg g-1 in 16 h, a time at which equilibrium was reached. At 25 °C, the eugenol decreased from 5.16 ± 0.78 mg g-1 to 0.26 ± 0.01 mg g-1, reaching equilibrium within 6 h, 10 hours earlier than at 5°C.
Difusion os eugenosl from the AAF1
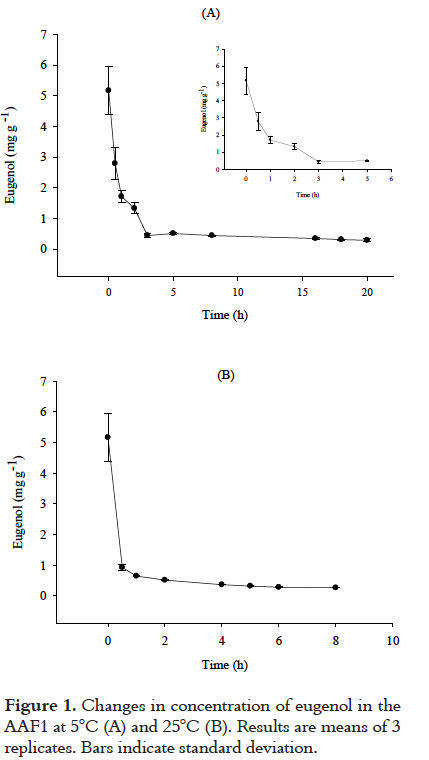
Figure 2 shows the diffusion graphs, following Fick's second law, for the release experiments carried out at 5°C and 25°C (AAF1). The D values were determined according to Eq. (1) as 2.04 ± 0.12 x 10-8 cm2 s-1 at 5°C and 7.89 ± 0.39 x 10-8 cm2 s-1 at 25°C. These values are within the same order of magnitude, but a higher D value was obtained at 25°C.
In vitro antibacterial activity of eugenol on P. fluorescens
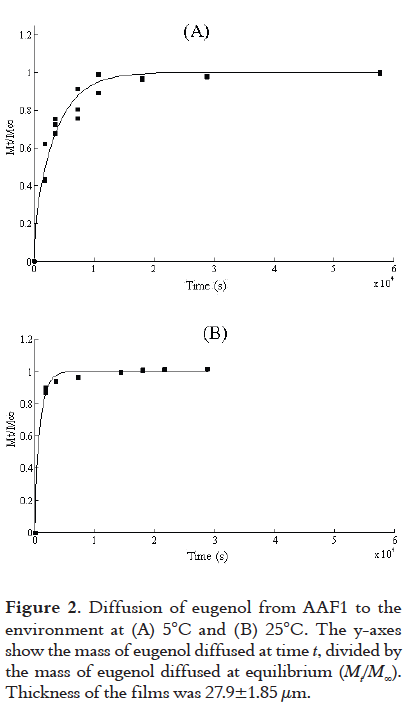
Eugenol inhibited the growth of P. fluorescens at all of the dilutions tested. The 1:1 dilution (1.74 mg eugenol per disc) presented the largest inhibition zone (21 mm), followed by 1:5 (0.87 mg eugenol per disc; 16-15 mm) and 1:10 (0.36 mg eugenol per disc; 10 mm considered the MIC). These results are summarized in Figure 3.
The control disc (without eugenol) presented no inhibition zone. Statistically significant differences (P < 0.05) were observed for the different dilutions.
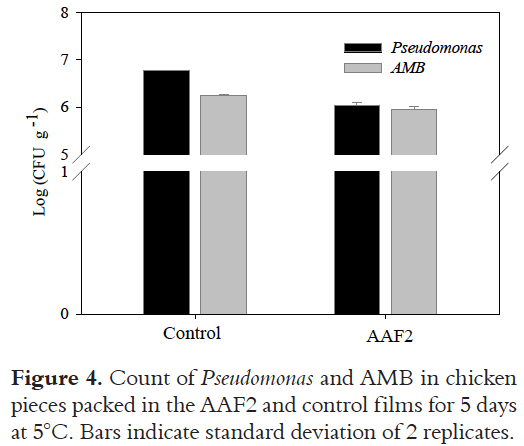
The effect of the AAF2 on fresh chicken pieces
The AAF2 was tested directly on fresh chicken pieces (thighs). The results of microbial counts of Pseudomonas ssp. and AMB at 5°C are summarized in Figure 4. The rationale for incorporating antimicrobial compounds into packaging is to prevent surface growth in foods where a high level of spoilage and contamination occurs. The presence of eugenol (4.87 mg g-1) in the AAF2, showed a small reduction of the number of Pseudomonas (1.1 x 106 CFU g-1) compared to the control film (6.0 x 106 CFU g-1), with statistically significant differences (P < 0.05) (6.3 log CFU g-1 and 6.77 log CFU g-1 respectively).
Thus, the presence of eugenol in the AAF2 caused it to be more effective than the control film. These results are consistent with those of the in vitro test, in which P. fluorescens was sensitive to the presence of eugenol (at concentrations as low as 0.36 mg eugenol per disc).
The effect of the AAF2 on the growth of AMB in fresh chicken thighs was better to reduce the microflora, from 1.7 x 106 CFU g-1 in the control film and 9.0 x 105 CFU g-1 in the film with eugenol (P < 0.05) (5 days at 5°C) (6.2 log CFU g-1 and 5.9 log CFU g-1 respectively). These bacterial counts found in the present work, are within the limits established by NOM-034-SSA1-1993 for 5 x 106 CFU g-1 in Mexico [37].
DISCUSSION
The high losses of eugenol during the manufacture of films were due to the high processing temperatures, which caused the rapid evaporation or decomposition of the eugenol. In addition, the losses may have been caused by the chemical instability of eugenol in the presence of air, light, oxygen and other environmental factors. Suppakul et al., [38] reported higher losses of antimicrobial agents, such as linalool and methylchavicol from LDPE and ethylene vinyl acetate (EVA) than those observed in the present study; after the blowing process, the authors observed a concentration of 0.34 g/100 g, down from an initial concentration of 1.0 g/100 g. Galloto et al., [39] reported that nearly 30% of the thymol initially included in a formulation of antimicrobial low density polyethylene (LDPE) film remained after the extrusion process. Yuwono et al. [27], reported that the vapor pressure of eugenol increases from 0.01 mm Hg at 20°C to 20 mm Hg at 138.7°C; therefore, losses of eugenol were influenced by evaporation into the environment due to the high volatility of eugenol at processing temperatures.
At the end of the diffusion experiments, close to 94% of eugenol had been released from both films. As the temperature increased, the time needed to cause the release of eugenol was diminished due to the fact that an increased temperature favored the evaporation of eugenol from the polymer matrix into the environment. These results suggest that the antimicrobial release began within the first minutes of the exposure of the films to the environment and quickly reached equilibrium. The results agree with those reported by Dhoot et al. [32], who reported that the steady state of the diffusion of eugenol through a linear low-density polyethylene (LLDPE) film was reached after 8.3 h at 16°C. The release of eugenol from the AAFs depends on factors such as temperature and polymer type. If exposed to the air, a vapor pressure of 0.03 mm Hg at 25°C [27] indicates that eugenol will exist solely as a vapor in the ambient atmosphere. Vapor-phase eugenol is degraded in the atmosphere by a reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 6 hours, a time that coincides with the release observed at the temperature of this study (25°C). The behavior of the AAFs shows that during their application as food packaging they will release eugenol under refrigerated conditions. In the case of temperature increases, the release rate will rise, increasing the antimicrobial activity, which will be needed due to the increased growth rate of microorganisms at higher temperatures.
Regarding the diffusion of eugenol from the AAF1, Kuorwel et al., [40], reported that increasing the temperature from 15 °C to 35 °C, the diffusion coefficient increased for carvacrol, linalool and thymol. Piringer and Baner [41], reported a D value of 1.3 x 10-10 cm2 s-1 for eugenol in LDPE films that were in contact with methanol/ethanol at 23°C. This value is lower than the values determined in the present work because they kept the polymer phase in contact with a methanol/ethanol liquid phase. The contact with organic liquids may have decreased the release of eugenol from the film. Diffusion of eugenol in LLDPE films was also determined by Dhoot et al. [32], who found a D value of 8.86 x 10-10 cm2 s-1 at 23°C. However, these authors reported the diffusion, or penetration, of pure liquid eugenol into the LLDPE matrix. Therefore, it seems that pure eugenol penetrates more slowly into a polymer matrix than it is released at a low concentration from a polymer into the environment. The fast migration of eugenol is due to its ability to diffuse through the LDPE chains to rapidly leave the system. It appears that there are no chemical interactions between the eugenol molecule and the non-polar chains of polyethylene. The use of such films could increase the shelf life of a foodstuff under refrigerated conditions by implementing a system with a slow migration of the agent from the packaging material onto the surface of the foodstuff. Encapsulation of eugenol in materials like β-cyclodextrin before its incorporation into the films is the next step in this research.
The control disc (without eugenol) presented no inhibition zone. This result confirmed the in vitro antimicrobial activity of eugenol, which showed a high inhibitory effect on P. fluorescens. Girova et al., [28], reported that clove oil, which is composed of 76.8% eugenol, showed antimicrobial activity against P. fluorescens. Ouattara et al. [29] reported that P. fluorescens was affected by a 1:100 dilution of clove oil. In both cases, the antimicrobial effect was attributed to the presence of eugenol. The mechanism of action of the antimicrobial effect of eugenol remains unclear. An important characteristic of eugenol is its hydrophobicity. This property allows it to penetrate lipopolysaccharide layer in the Gramnegative bacterial cell membrane and to disturb cellular structures [25]. It has been suggested that this activity is caused by the presence of phenolic compounds and monoterpenes [20]. The phenolic components weaken the phospholipid bilayer of the cell membrane, causing an increase in permeability and leakage of the intracellular components [17]. Pandima et al., [42] reported that primary mechanism of action of eugenol is disruption of the cytoplasmic membrane, which increases its non-specific permeability. This hyperpermeability is followed by leakage of ions and extensive loss of other cellular contents, including the intracellular proteins and ultimately results in cell death.
Han and Floros [43], reported that during an extrusion, the high temperature conditions in the extruder affected the chemical stability of incorporated antimicrobial substances and reduced their residual antimicrobial activity. The results of the present study showed that the AAF2 films showed moderately reduction of growth of Pseudomonas and AMB when they were used to package chicken thighs. Eugenol, by its nature, is highly soluble in the fatty chicken skin, which promotes its diffusion and action on the surface of the chicken pieces. Another possible explanation is the effect of differences between the diffusion rate of the antimicrobial agent and the growth rate of the microorganisms. The antimicrobial agent could be depleted and lose the antimicrobial activity of the film before the growth of the microorganisms increases. Also due to the release of eugenol from the AAF2, perhaps the remaining amount was actually below the MIC, insufficient to inhibit growth of the bacteria studied. Based on P. fluorescens growth curve (not shown), the log phase started after 7.5 hours of incubation (26°C). By that time, most of the eugenol had already been released from the AAF (25°C). Therefore, the antimicrobial activity of AAF depends on the relationship between the release rate of eugenol from the film and the growth rate of bacteria.
Despite the losses of eugenol during the processing of the AAF, and the rapid release from the film at 5°C and 25°Â°C, AAF2 was effective in retarding the growth of Pseudomonas and AMB in chicken thighs. The release of the antimicrobial agent was directed towards the food surface, which allowed relatively low concentrations of eugenol in the LDPE film to maintain the microbial quality of chicken.
In order to minimize the losses of eugenol during the production of the AAFs, research on the encapsulation of eugenol in β-cyclodextrin and its incorporation into multilayer films are currently under way. Future studies will also evaluate the effect of this antimicrobial agent on the sensory properties of chicken.
CONCLUSIONS
LDPE films supplemented with 9.0 and 7.7 mg g-1 eugenol were manufactured, and 42.6% and 36.7% of the eugenol, respectively, were lost during the production process. The release of eugenol from the films into the environment showed Fick's behavior, with D values of 10-8 cm2 s-1 at 25°C and 5°C (same order of magnitude). At 25°C the equilibrium was reached within 6 h, 10 h earlier than 5°C. Despite the losses of eugenol during the extrusion of the films, they showed an antimicrobial effect during contact with fresh chicken by reducing the growth of Pseudomonas and AMB. Therefore, the addition of eugenol to LDPE showed a potential to maintain the chicken quality in food packaging applications.
AKNOWLEDGEMENTS
We gratefully acknowledge Q.B. Rosalva Pérez-Morales for technical assistance with the microbiological studies. This study was supported by funds from/CONACYT/FOMIX (SON-2008- CO3-106207) and Qualyplast, S.A. de C.V.
CONFLICT OF INTERESTS
The authors declare that no conflict of interest exist for the present work.
REFERENCES
1. SAGARPA. Situación y perspectiva de la producción de la carne en México. Disponible en: http://www.sagarpa.gob/ganaderia. [ Links ]
2. Avicultura.com. La producción de carne de ave crecerá en la UE hasta las 13,6 millones de toneladas en 2023. Disponible en: http://www.avicultura.com/2014/02/24/la-produccionde- carne-de-ave-crecera-ue-hasta-las-millones-de-toneladasen- 2023/. [ Links ]
3. Tendencias Avícolas Mundiales: El consumo de pollo en América casi triplica el promedio mundial. http://www.elsitioavicola.com/ articles/2043/tendencias-avacolas-mundiales-el-consumo-depollo- en-amarica-casi-triplica-el-promedio-mundial/#sthash. EWK4GlbJ.dpuf. [ Links ]
4. Tendencias Avícolas Mundiales: Mayor consumo de pollo fuera de la UE. Disponible en: http://www.elsitioavicola.com/articles/ 2471/tendencias-avacolas-mundiales-2013-mayor-consumo- de-pollo-fuera-de-la-ue/#sthash.19cGIVqg.dpufTendencias avícolas mundiales. 2013. [ Links ]
5. Jiménez SM, Salsi MS, Tiburzi, MC, Rafaghelli RC, Tessi MA, Coutaz VR. Spoilage microflora in fresh chicken breast stored at 4°C: Influence of packaging methods. J Appl Microbiol. 1997; 83: 613-618. [ Links ]
6. Shin J, Harte B, Ryser E, Selke S. Active packaging of fresh chicken breast, with allyl isothiocyanate (AITC) in combination with modified atmosphere packaging (MAP) to control the growth of pathogens. Food microbiology and safety. J Food Sci. 2010; 75: 65-71. [ Links ]
7. Arnaut-Rollier I, Vauterin L, De Vos P, Massart DL, Devriese LA, De Zutter L, Van Hoof J. A numerical taxonomic study of the Pseudomonas flora isolated from poultry meat. J Appl Microbiol. 1999; 87: 15-28. [ Links ]
8. Hotchkiss JH. Current and future trends in active food packaging. In Proceedings of the II Food Packaging International Congress RISEA-2000. Editor H. Soto-Valdez. Hermosillo, Sonora, México. 2000. 39-51 p. [ Links ]
9. Ahvenainen R, Hurme E. Active and smart packaging for meeting consumer demands for quality and safety. Food Addit Contam. 1997; 14: 753-763. [ Links ]
10. Vermeiren L, Devlieghere F, van Beest M, de Kruijf N, Debevere J. Developments in the active packaging of foods. Trends Food Sci Tech. 1999; 10: 77-86. [ Links ]
11. Crank J. The mathematics of diffusion (2nd ed.). Oxford, UK: Oxford Science Publications. 1975. [ Links ]
12. Cooksey K. Utilization of antimicrobial packaging films for inhibition of selected microorganism. In ACS symposium series. Food packaging. Washington, DC: American Chemical Society. 2000. 17-25 p. [ Links ]
13. Jayasena DD, Cheorun J. Essential oil as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci Tech. 2013; 34: 96-108. [ Links ]
14. Ramos M, Jiménez A, Peltzer m, Garrigós MC. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J Food Eng. 2012; 109:513-519. [ Links ]
15. Irkin R, Esmer OK. Novel food packaging system with natural antimicrobial agents. J Food Sci Technol. 2015. DOI 10.1007/ s13197-015-1780-9 [ Links ]
16. Sanla N, Jangchud A, Chonhenchob V, Suppakul P. Antimicrobial activity of cinnamaldehyde and eugenol and their activity after incorporation into cellulose-based packaging films. Packag Tech Sci. 2012; 25:7-17. [ Links ]
17. Ouattara B, Simard RE, Piette G, Bégin A, Holley RA. Diffusion of acetic and propionic acid form chitosan-based antimicrobial packaging films. Food chemistry and toxicology. J Food Sci. 2000; 65: 768-773. [ Links ]
18. Sung SY, Lee TS, Tee TT, Bee ST, Rahmat AR, Rahman WAWA, Tan, Ach, Vikhraman M. Antimicrobial agents for food packaging applications. Review. Trends Food Sci Tech. 2013; 33: 110-123. [ Links ]
19. Celikel N, Kavas G. Antimicrobial properties of some essential oils against some pathogenic microorganisms. Czech J Food Sci. 2008; 26: 171-181. [ Links ]
20. Friedman M, Henika P, Mandrell R. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes and Salmonella enterica. J Food Protect. 2002; 65: 1545-560. [ Links ]
21. López P, Sánchez C, Batlle R, Nerín C. Development of flexible antimicrobial films using essential oils as active agents. J Agr Food Chem. 2007; 55: 8814-8818. [ Links ]
22. Becerril R, GÓmez-Lus P, Goñi P, López P, Nerín C. Combination of analytical and microbiological techniques to study the antimicrobial activity of a new active food packaging containing cinnamon or oregano against E. coli and S. aureus. Anal Bioanal Chem. 2007; 388: 1003-1011 [ Links ]
23. Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essentials oils. BMC Complem Altern M. 2006; 6: 39-46. [ Links ]
24. Tippayatum P, Chonhenchob V. Antibacterial activities of thymol, eugenol and nisin againts some food spoilage bacteria. Kasetsart Journal (Nat. Sci.) 2007; 41: 319-323. [ Links ]
25. Burt S. Essential oils: Their antibacterial properties and potential applications in food- a review. Int J Food Microbiol. 2004; 94: 223-253. [ Links ]
26. Saïdana D, Mahjoub S, Boussaada O, Chria J, Mahjoub MA, Chérif I, Helal AN. Antibacterial and antifungal activities of the essentials oils of two saltcedar species form Tunisia. J Am Oil Chem Soc. 2008; 85: 817-826. [ Links ]
27. Yuwono M, Siswandono Hafid AF, Poernomo AT, Agil M, Indrayanto G, Ebel S. Eugenol. Analytical profiles of drug substances and excipients. London, UK. Academic Press. Vol. 27. In H. Brittain (Ed.). 2002; 149–177 p. [ Links ]
28. Girova T, Gochev V, Jirovetz L, Buchbauer G, Schmidt E, Stoyana A. Antimicrobial activity of essential oils form spices against psychrotrophic food spoilage microorganism. Biotechnol Biotec Eq. Special Edition. 2010; 547-552. [ Links ]
29. Ouattara B, Simard RE, Holley RA, Piette GJP, Bégin A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int J Food Microbiol. 1997; 37: 155-162. [ Links ]
30. Manzanarez-López F, Soto-Valdez H, Auras R. Peralta E. Release of a-tocopherol from poly(lactic acid) films, and its effect on the oxidative stability of soybean oil. J Food Eng. 2011; 104: 505–517. [ Links ]
31. Soto-Valdez H, Auras R, Peralta E. Fabrications of poly(lactic acid) films with resveratrol and diffusion to ethanol. J App Polym Sci. 2011; 121: 970-978. [ Links ]
32. Dhoot G, Auras R, Rubino M, Dolan K, Soto-Valdez H. Determination of eugenol diffusion through LLDPE using FTIR-ATR Flow Cell and HPLC Techniques. Polymer. 2009; 50: 1470-1482. [ Links ]
33. Mann CM, Markham JL. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol. 1998; 84: 538-544. [ Links ]
34. Hintze JL. NCSS. Quick start and self help manual. Statistical system for windows. Kaysville, UT: NCSS. 2007. [ Links ]
35. NOM-092-SSA1-1994. Norma Oficial Mexicana. Bienes y servicios método para la cuenta de bacterias aerobias en placa. [ Links ]
36. NOM-110-SSA1-1994. Norma Oficial Mexicana. Bienes y servicios. Preparación y dilución de muestras de alimentos para su análisis microbiológico. [ Links ]
37. NOM-034-SSA1-1993. Norma Oficial Mexicana. Bienes y servicios. Productos de la carne, carne molida y carne molida moldeada. Envasadas. Especificaciones sanitarias. [ Links ]
38. Suppakul P, Sonneveld K, Bigger SW, Miltz J. Loss of AM additives from antimicrobial films during storage. J Food Eng. 2011; 105: 270-276. [ Links ]
39. Galotto MJ, Valenzuela X, Rodríguez F, Bruna J, Guarda A. Evaluation of the effectiveness of a new antimicrobial active packaging for fresh atlantic salmon (Salmo Salar L.) Shelf life. Packag Technol Sci. 2012; 25(6): 363-372. [ Links ]
40. Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW. Migration of antimicrobial agents from starch-based films into a food simulant. LWT Food Sci Tech. 2013; 50: 432-438. [ Links ]
41. Piringer OG, Baner AL. (Eds.). Plastics packaging materials for food. Weinheim, Germany: Wiley-VCH. 2000. [ Links ]
42. Pandiva DK, Arif NS, Sakthivel R, Karutha PS. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J of Ethnopharmacol. 2010; 130: 107–115. [ Links ]
43. Han JH, Floros JD. Modeling antimicrobial activity loss of potassium sorbate againts Baker´s yeast after heat process to develop antimicrobial food packaging materials. Food Sci Biotechnol. 1999; 8: 11-14. [ Links ] 1998; 84: 538-544.