Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.22 no.2 Medellín May/Aug. 2015
https://doi.org/10.17533/udea.vitae.v22n2a09
DOI:10.17533/udea.vitae.v22n2a09
REVIEWS
CONTENT OF Hg, Cd, Pb AND As IN FISH SPECIES: A REVIEW
CONTENIDO DE Hg, Cd, Pb Y As EN ESPECIES DE PECES: REVISION
Julián ZULUAGA RODRÍGUEZ1*, Sara Elisa GALLEGO RÍOS1, Claudia María RAMÍREZ BOTERO1
1 Grupo Impacto de los Componentes Alimentarios en la Salud ICAS, Escuela de Nutrición y Dietética, Universidad de Antioquia UdeA, Medellín, Colombia
* Corresponding author: julian.zuluagar@udea.edu.co
Recibido: Marzo 15 de 2015
Aceptado: Septiembre 21 de 2015
ABSTRACT
Background: One of the main water contaminants are Hg, Cd, Pb and As. The chain of contamination of these metals and metalloid follows a cyclical order: Industry, atmosphere, land, water, phytoplankton, zooplankton, fish, and humans. Currently, Hg, Cd, Pb and As researches are of interest because consumption of fish with high toxic metal and metalloid concentrations affects human health. Objectives: Provide information on the characteristics of Hg, Cd, Pb and As in the problematic of fishing resource contamination, their implications on human health, and international evidence on studies conducted on Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, thunnus and Megalops fish genera. Methods: Database, Science Direct, Pub Med, Escopus, Springer Link, and Scopus, available information was reviewed using the keywords: Heavy metals, water pollution, fish, mercury, cadmium, lead, arsenic, health risk, regulations, biomagnification, and bioaccumulation. Results: The metals that pose the highest risks for human health are mercury, cadmium, lead, and arsenic which cause important complications in the nervous system, kidneys, bones, lungs, and cardiovascular system due to their toxicity and possible carcinogenic effect. Fish contents of Hg, Cd, Pb and As varies depending on the zone, environmental conditions, the contamination level of the fishing site, and the characteristics of the fish, being some fish more prone to accumulating higher concentrations of these metals in their muscles; among the species, the metal that showed the highest risk was mercury, being in high concentrations in the largest, most enduring predatory fish. Conclusions: Studies submitted in this review, may be used as the base for future comparisons with Hg, Cd, Pb and As concentration values in different fish studies for the Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, and Megalops genera in order to be able to determine consumption recommendations and warnings.
Keywords: Fishes, Water pollution, Mercury, Cadmium, Lead, Arsenic
RESUMEN
Antecedentes: Uno de los principales agentes contaminantes en las aguas son Hg, Cd, Pb y As. La cadena de contaminación de estos metales sigue un orden cíclico: industria, atmósfera, suelo, agua, fitoplancton, zooplancton, peces y humanos. Las investigaciones en Hg, Cd, Pb y As son de interés en la actualidad ya que el consumo de pescado con altas concentraciones de estos metales y metaloides tóxicos afecta la salud humana. Objetivos: Ofrecer información de las características del Hg, Cd, Pb y As en la problemática de contaminación de los recursos pesqueros, sus implicaciones para la salud humana y evidencias internacionales de los estudios realizados en los peces de los géneros Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus y Megalops. Métodos: Se revisó información disponible en las bases de datos, Science Direct, Pub Med, Springer Link, Scopus, utilizando las palabras clave: heavy metals, water, fish, mercury, cadmium, lead, arsenic, health risk, regulations, biomagnification, bioacumulation. Resultados: Los metales con mayor riesgo para la salud humana son mercurio, cadmio, plomo y arsénico, los cuales causan complicaciones importantes en el sistema nervioso, renal, óseo, pulmonar, cardiovascular debido a su toxicidad y posible efecto carcinogénico. El contenido de Hg, Cd, Pb y As en el pescado varía dependiendo de la zona, las condiciones medioambientales, el grado de contaminación del lugar de pesca y las características del pez, siendo algunos peces más propensos a acumular concentraciones más elevadas de estos metales en el músculo, entre las especies de peces, el metal que mostro mayor riesgo fue el mercurio, estando en altas concentraciones en los peces más grandes, de mayor longevidad y depredadores. Conclusiones: Los estudios presentados en esta revisión pueden servir como base para futuras comparaciones con valores de concentración de metales pesados en diferentes estudios de peces para los géneros Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus y Megalops, con la finalidad de poder establecer recomendaciones y advertencias sobre su consumo.
Palabras clave: Peces, contaminación del agua; mercurio; cadmio; plomo; arsénico
INTRODUCTION
Currently, chemical products coming from sources like industrial, urban, and agricultural waste discharges are contaminants of the surfaces and sediments of the waters in the world (1). One of the main contaminant agents in the waters are Mercury (Hg), Cadmium (Cd), Lead (Pb), and Arsenic (As). The chain of contamination of these metals and metalloid follows a cyclical order: Industry, atmosphere, land, water, phytoplankton, zooplankton, fish, and humans (2). In some fish species, contamination levels are so high, they may cause adverse effects on human health. Some countries issue alerts on the risk of contaminated fish consumption; however, they are ignored by public at large who continue consuming without taking into account the serious health consequences (3).
Trace elements are present in every ecosystem in the world (4); nevertheless, the increase of these metals and metalloids in marine systems has become a risk to human health due to their toxic effects (5). The main Hg, Cd, Pb and As contamination factors in fluvial and marine sources are industrial and municipal wastewater discharge, mining, combustion of fossil fuels, deforestation and fertilizers used in agriculture (6–8). Also natural sources such as soil weathering and volcanic activity pollute the environment significantly (9). Trace elements can be soluble in water and react with organic matter forming complexes and chelates, which increase its solubility, availability and dispersal (10).
Once trace elements are released into the waters, they bioaccumulate into aquatic sediments depending on environmental conditions water cycle, seasonal variations, pH, microorganisms, sediment reduction and oxidation potential (11–15); in the sediments takes place the migration of metal compounds from the abiotic environment into aquatic organisms and the subsequent introduction and bioaccumulation in marine food chains (16), predators exhibit highest concentrations (17). Accumulation of metal in the tissues organism depends mainly on water concentrations, bioavailability and fish trophic position (18, 19).
The main metal threats to human health have been mainly associated to Hg, Cd, Pb, and As exposure (20, 21); they get into humans through diet, which poses a risk for populations with fish consumption over 8 – 12 ounces per week, exceeding the United States Environmental Protection Agency (U.S. EPA) recommendations (22, 23).
Hg has a series of chemical transformations in the environment, appearing as zero oxidation state (Hg0), mercurous state (Hg+), and mercuric state (Hg+2); additionally, it may form organic compounds, being methylmercury (MeHg), the most important form in terms of toxicity and effects on health (5). MeHg causes damages at the central nervous system level and its neurotoxic effect is attributed to mitochondrial damage via glutathione reduction (GSH), which reduces ATP synthesis and increases lipid, protein, and DNA peroxidation; MeHg, due to its high affinity with the sulfhydryl group, it forms complexes with N-acetylcysteine and cysteyne, important precursors of GSH, which increases free radical production and reduces the oxidation defense systems and the imbalance between both processes produces the uncontrolled release of calcium from the mitochondrion, disturbing intracellular calcium levels (24, 25).
Cd accumulates in the human body for long periods of time causing damages to the nervous system, kidneys, bones, lungs, and cardiovascular system (26, 27), Cd cytotoxicity induces cellular dysfunctions, including cell death, reducing DNA breathing, and increasing mutagenesis. It has been classified as carcinogenic for humans (Group 1) (28), in spite of it not being directly mutagenic. DNA damages that take place after exposure to cadmium seem to be mainly mediated by the indirect production of free radicals, partly due to the inhibition of cellular antioxidants and oxidative stress increase (29).
Pb is neurotoxic and, due to its capacity to join erythrocytes, it may be widely distributed to the human body organs where it concentrates and generates damage. Lead toxicity is due to its ability to substitute itself with other divalent cations, specially calcium and zinc, affecting their functions into the cell, mainly in the cellular organelles like the mitochondrion, where its concentration affects the energetic metabolism and favors the generation of free radicals. One of the reasons why lead may substitute itself with diverse cations in the cell is its ability to form stable interactions with oxygen and sulfur, common components in the sites where proteins join the metal, and, besides, due to its longer ionic range and higher electronegativity lead is more related to these proteins than other cations like calcium and zinc; however, lead charge distribution is irregular due to the presence of two inert electrons in its electron cloud, which alters the structure and function of the joined protein, inhibiting its functionality (30, 31).
Chronical exposure to As, has been associated to skin injury, peripheral neuropathy, encephalopathy, hepatomegaly, cirrhosis, hematite metabolic alterations, bone marrow depression, diabetes, and renal failure. The action mechanisms of this metal depend on the way they present. In its pentavalent form, as arsenate (As+5), it may replace phosphorus in multiple biochemical reactions having as the main consequence ATP depletion. In its trivalent form, arsenite (As+3), it has a great affinity with the thiol groups, turning it into a powerful GHS inhibitor, and thioredoxin reductase, which may alter the cellular oxide reduction mechanisms generating cytotoxicity. As+3 it is also a pyruvate dehydrogenase (PDH) inhibitor, which ultimately reduces ATP production (32, 33).
There are important precedents on the serious consequences of Hg, Cd, Pb and As contaminated fish consumption has on human health, being worth mentioning: Minamata (Japan) tragedy, where over 900 people lost their lives and two million suffered health problems as a consequence of having eaten Hg contaminated fish, Minamata disease (34), and the event at Jinzu River basin, at Toyama prefecture, where one of the most serious Cd prolonged intake intoxication cases takes place following the mining extractions that contaminated those waters (35). In South America, an anthropogenic source contamination increase has been evidenced in high concentrations of trace elements on fish tissues (36). In Colombia, there are studies on biota metal concentration determination studies, such as those conducted by Olivero and Johnson at Ciénaga Grande de Achi, located on Caribona River basin, Cauca River sub-basin; Ciénaga de Simití, east of San Lucas mountain range, on Magdalena River; and south of Bolívar, Magdalena and Cauca lowland swamps, on species characteristic of these regions (37). However, there are no reports of importance on the Colombian Caribbean, on Hg, Cd, Pb and As contamination fishing resources, like porgy, coastal trevally, jack mackerel, wahoo, skipjack tuna, mud sucker, grouper, tuna and gropers, among others, belonging to the genera Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, thunnus and Megalops (38); which are of commercial importance in the market of this region (39). Therefore, it is required to conduct studies to determine fish Hg, Cd, Pb and As content in this region. The objective of this work was to submit a review of the characteristics of Hg, Cd, Pb and As in the contamination problematic of fishing resources, their implications on human health, and international evidence of studies conducted on fish belonging to genera (Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, Thunnus and Megalops).
MATERIALS AND METHODS
A bibliographical review of information between 2001 and 2015 was performed along some classical articles about Mercury, Cadmium, Lead, and Arsenic contents in Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, Thunnus and Megalops genera that share characteristics with the fish of the same genera found in the Colombian Caribbean; those researches that quantified wet weight (mg/kg o µg Hg /g wet weight) or if this information was possible to be extracted from the article were selected. These metals were also characterized and articles about the implication of consumption on health were taken into account. The regulations stipulated by Codex Alimentarius and Joint FAO/WHO Expert Committee on Food Additives (JECFA) were considered to analyze the maximum metal content allowable limits in fish and fish existing tolerable provisional intake recommendations to guarantee safe consumption. Documents were selected from databases, Science Direct, Pub Med, Escopus, Springer Link, and Scopus using the following keywords for the search: Heavy metals, water contamination, fish, mercury, cadmium, lead, arsenic, health risk, regulations, biomagnification, and bioaccumulation (Figure 1).
RESULTS
Mercury
Hg is a transition element of Group 12 (II B), with an atomic mass of 200.59 amu and it is one of the most toxic metals in aquatic ecosystems. A series of complex chemical transformations allows mercury to be present in the environment in its three oxidation states (5). Atmospheric Hg in the form of mercury vapor (Hg0), is derived from soil weathering, volcanic activity and ocean evaporation. Also anthropogenic sources like refining and combustion of fossil fuels, gold mining, chlor alkali industry, cement, steel and phosphate production; the release of Hg in the world are estimated in 6000 tons per year, in Colombia it is estimated that each year around 31.26 tons are released into the water (40). In the environment Mercury vapor it is oxidized to a water-soluble inorganic form (Hg+2), the metal may then be reduced back to Hg0, or it may be methylated by microorganisms present in the freshwater and oceanic sediments, this biomethylation reaction produces MeHg, the mainly form of mercury in aquatic organism. MeHg into aquatic food chains follows the cycle, plankton, herbivorous fish and carnivorous fish (5,41).
Gastrointestinal absorption of Hg+2 form compounds in food is around 15%, while MeHg absorption is 90 to 95%; generally, about 80 to 100% of Hg found in fish muscle corresponds to MeHg (41). The biological average life of Hg is estimated at about 44 days (5).
The toxicological profile of Hg varies depending on its form. Exposure to Hg0 and MeHg produce symptoms in the central nervous system, while Hg mono and divalent forms act mainly in the kidney. MeHg corresponds to the most toxic organic form of Hg (25). It is neurotoxic due to its accumulation in the central nervous system, deteriorating physiological functions through the interruption of the endocrine glands (42). MeHg is classified as carcinogenic for humans, group 2B, mainly related to liver and esophagus cancer (28). In addition, methylmercury can cross the placenta causing neurotoxic effects during human brain development, probably permanent (43, 44).
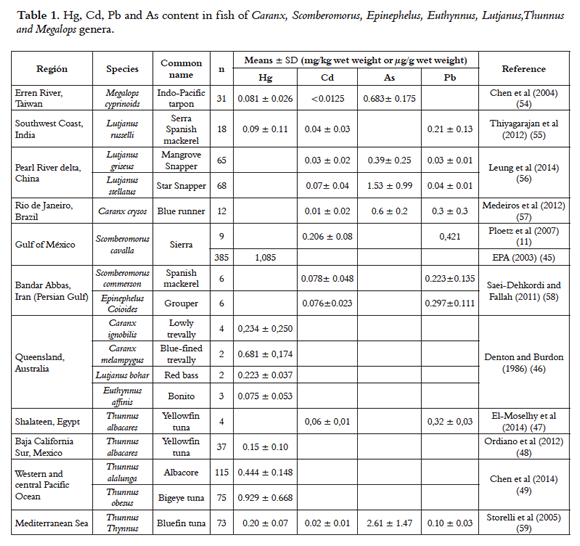
Some authors have reported the content of Hg in fish (Scomberomorus cavalla, Caranx ignobilis, Caranx melampygus, Lutjanus bohar, Euthynnus affinis, Lutjanus russelli, Thunnus albacores, Thunnus alalunga, Thunnus obesus, Thunnus Thynnus and Megalops cyprinoids, Table 1), in the Gulf of Mexico, Queensland, Australia, southeast of Taiwan, western and central Pacific Ocean, Mediterranean Sea and southwest coast of India (45–49) finding Hg levels in muscle over 0.5 µg Hg/g wet weight for the largest, most enduring predatory fish (Scomberomorus cavalla 1.085 µg Hg/g wet weight, Caranx melampygus 0.681 ± 0,174 µg Hg/g wet weight, see Table 1. According to what has been reported in Queensland, the highest Hg levels are mainly due to biomagnification of the food chain of this metal (46). These findings are congruent with data reported by other researchers on these fish species that have similar characteristics (50–52).
An epidemiologic study conducted in San Francisco was able to demonstrate that the consumption of predatory fish, which may have high Hg levels in its MeHg form entails adverse health effects. The study determined a significant correlation of high levels of mercury on subjects who consumed swordfish 1.94 times a month, in average, using as reference, 150-277 gram portions. This research analyzed blood Hg levels on 89 participants who consumed more than 30 species of fish and 82 of them showed levels over 5 µg Hg/L in the blood and 16 showed levels over 20 µg Hg/L in the blood (US EPA and the National Academy of Sciences recommend keeping blood mercury levels under 5 µg/L) (53).
In Colombia, Hg contamination is mainly associated to mining activity, combustion of fossil fuels, waste incineration, waste disposal, waste water, where the metal is released into the water and atmosphere contaminating fish living in those areas (60). A study conducted on hair samples of 1,328 individuals, located along Cauca and Magdalena rivers, where most mining activities take place, showed that individual hair Hg average was 1.560 ± 0.060 µg/g, with values oscillating between 0.010 and 20.140 µg Hg/g in hair; 52.0% of the studied population exceeded the 1.0 µg Hg/g in hair recommendation determined by USEPA. The habitants located closer to the mining areas were those who showed the highest Hg level in hair; in this study, 99.8% consumed fish at least once a day and in some cases the intake was three times a day (61). Another study conducted at Caimito, a town near San Jorge River, located in the northwestern region of Colombia, apparently free from mining activities, hair mercury levels were measured to 94 participants between 15 to 65 year-old, finding an average Hg concentration of 4.910 ± 0.550 µg Hg /g in hair; no significant differences were found on Hg levels in hair by sex or age but, however, a small but significant correlation was found between fish consumption frequency and Hg levels in hair (62).
Due to Hg intake consequences on health, the Codex Alimentarius determines maximum allowed levels of MeHg in fishing products and fish meat of 0.5 µg MeHg/ g, excepting predatory fish, where the maximum allowed level is 1.0 µg MeHg/ g; additionally, the Codex also counts on an inorganic Hg Provisional Tolerable Weekly Intake of 4 µg/ kg of body weight for humans and MeHg of 1.6 µg/ kg of body weight (63). In 2007, JECFA reaffirmed the existing PTWI of 1.6 µg /kg of body weight for MeHg, based on the toxicological critical point (neurotoxicity development) in the human species (64).
Cadmium
Cd is an element of the Group 12 (II B), with an atomic weight of 112.40 amu and it is issued to the ground, water, and air through mining, non-ferrous metal refining, manufacturing and application of phosphate fertilizers, fossil fuel burning, and waste incineration and disposal. There is evidence that about 30,000 tons of cadmium are released into the environment every year with an estimated 4,000- 13,000 tons from human activities affecting aquatic organisms,The average level of cadmium in ocean water has been reported between < 5 and 110 ng/L (65). The nutrient-like profiles of Cd and correlation whit the phosphate in the ocean indicate its uptake by phytoplankton. Cd is then transferred to zooplankton and upper trophic levels through food chain webs; however the exact mechanisms of Cd uptake and the relationship with phosphates and other nutrients remain unknown (66-68). Human beings absorb Cd 5 to 8%, factor that is favored with low iron, calcium, and protein diets. Cd is transported by blood and distributed mainly to the liver and kidney (5) where it is long-term stored in the organism, having an average biological life of 17 to 30 years in human beings. Cd negatively affects the kidney, inducing tubular kidney failure and chronic kidney failure; in the lungs, it causes fibrosis (65), cardiovascular system risks due to the increment of cholesterol and free fatty acids in the blood, increasing the risk of aortic and coronary atherosclerosis (69), although the mechanisms and Cd relation to dyslipidemia have not been elucidated yet; studies suggest it is mainly due to the reduction of the High Density Lipoproteins (HDL) and to the high triglyceride and HDL proportion. Furthermore, Cd also affects children central nervous system causing neurological disorders, learning problems, and hyperactivity (70, 71). Some studies on adults and children's exposed to cadmium have suggested abnormal behavior and decreased intelligence, however because to the blood- barrier protection the direct toxic effect to occur only with cadmium exposure prior to blood-brain barrier formation or with blood-brain barrier dysfunction (5). It has been classified as carcinogen for group 1 humans and it has been mainly related to lung, prostate, pancreas, kidney, and bladder cancer (28). Several studies conducted in the US, Trinidad, Brazil, China, Iran, India, Egypt, Mediterranean Sea and Taiwan, demonstrated that the Cd concentrations in fish muscle were usually low. Among S. Commerson, E. Coioides, C. crysos, L. griseus, L. stellatus, T albacares, T thynnus and M. cyprinoids species, cadmium in muscle was generally lower than 0.1 µg Cd/g of wet weight (46,54-56,58), excepting the Gulf of Mexico, where Cd content in muscle was 0.206 ± 0.08 µg Cd/g of wet weight in S. cavalla, although this study found a significant correlation between the size of S. cavalla and liver Cd levels; a correlation between the size of S. cavalla and muscle Cd content was not found (11). The study conducted by Saei-Dehkordi and Fallah (58) in the Persian Gulf, found differences between the muscle Cd concentrations in S. Commerson during summer and winter seasons, 0.053 ± 0.035 and 0.102 ± 0.048 µg Cd/g of wet weight, respectively; the high Cd muscle levels during the winter are attributed to metal precipitation in the water due to the rainy season (58, 72).
Due to health consequences of Cd intake, Codex Alimentarius and JECFA determined a human Provisional Tolerable Monthly Intake (PTMI) of 25 µg Cd/kg of body weight (63, 73).
Lead
Pb is a Group 14 element (IV A), with an atomic weight of 207.2 amu; it exists in the Pb0, Pb+2, and Pb+4 oxidation states, generally in combination with two or more elements to form compounds (7); it gets to the aquatic system due to the ground superficial erosion and atmospheric deposition (74). Environmental levels have increased over 1,000 times in the last three centuries as result of human activity; the highest increase has taken place between 1950 and 2000 (7). Speciation of lead in marine waters is largely influenced by carbonates, chlorates and organic natural ligands. The proportion of inorganic complexes of lead is largely determined by the pH of the water, the concentration of these complex gradually increase with increasing total metal loading in sediments which suggest a potential threat to benthic organisms and aquatic biota in the marine system (75-77). Once absorbed, it is transported in the bloodstream to other tissues and it is accumulated in high concentrations in bones, teeth, liver, lung, kidney, brain, and spleen going through the blood-brain and placental barrier (5). Pb average biological life may be considerable higher in children than in adults; in the blood, it has an estimated average life of 35 days; in soft tissue, of 40 days; and in bones, 20 - 30 years (78). The main way of absorbed Pb excretion is the urinary tract, in general, with glomerular kidney filtration; it is also excreted with the bile through the gastrointestinal tract (5). The most affected systems by Pb are the nervous, the cardiovascular, hematologic, and renal. Lead poisoning symptoms are headaches, irritability, abdominal pain, and others related to the nervous system (20). Pb chronic toxicity in humans frequently produces apathy, irritability, low attentional capacity, epigastric pain, constipation, vomit, convulsions, coma, and death. In children, it may present encephalopathy with lethargy, mental dullness, vomit, irritability, and anorexia; in the most serious cases, prolonged exposure to Pb may reduce the cognitive function and cause conduct disorders, especially aggression, psychosis, confusion, and mental deficit (7). Pb is one of the contaminants consumed in diet that has been clearly identified as a risk for human health (79), it has been classified as carcinogenic for 2B Group humans while inorganic lead compounds have been classified as carcinogenic for 2A Group humans, mainly related to stomach cancer (28).
Often, high Pb concentrations in fish take place in areas close to mining activities and in areas with a high presence of this metal industry (80). Several studies have examined Pb concentrations in fish in the Gulf of Mexico, Iran, Brazil, China, Egypt, Mediterranean Sea and India; the highest levels have been reported by Ploetz et al. (11) in the Gulf of Mexico (0.421 µg Pb/g wet weight) in S. cavalla. Saei-Dehkordi and Fallah (58) in the Persian Gulf found lead concentrations in S. commerson and E. coioides that oscillate between 0.158 and 0.367 µg Pb/g wet weight; this study measured Pb concentrations in fish both in the winter and in the summer. Both species S. commerson and E. coioides, showed higher lead levels during the winter, 0.289 and 0.367 µg Pb/g, respectively. Medeiros et. al. (57), report levels of 0.3 µg Pb/g wet weight in the species C. crysos. The lowest Pb levels were found in fish in the Pearl River delta (China) in the species L. griseus and L. stellatu, with 0.03 and 0.04 µg Pb/g wet weight, respectively, in spite of it being a highly contaminated area by human activities (56).
Due to health consequences of Pb intake, Codex Alimentarius determines a maximum limit of Pb in fish of 0.3 mg/kg of wet weight (63). The current PTWI recommendation in humans of 25 µg Pb/kg of wet weight determined by JECFA has been withdrawn; the same committee, after assessing it in 2011, set forth it is not possible to determine a new PTWI that may be considered health protector (81).
Arsenic
As is a Group 15 element (V A), with an atomic weight of 74.922 amu found highly distributed in a natural way on the earth's crust; it may exist in the As+3 and As+5 oxidation states in a wide number of inorganic and organic forms with different toxicity levels (8). As anthropogenic sources include pesticides, wood and industry preservatives, mining and smelting wastes (32). As dissolves easily in marine waters where is present in trivalent, pentavalent and methylated forms. In anoxic conditions, Arsenate (As+5) is the dominant dissolved species, in surface water the arsenate is uptake by phytoplankton together with phosphate and transferred to arsenite (As+3) and methylarsenate and dimethylarsenate. Other organoarsenic compounds of arsenate and arsenite like monomethylarsonic acid (MMA) dimethylarsinic acid (DMA), arsenobetaine (Ab) are produced by microbiological processes in the sediments (82,83). Arsenobetaine is considered the dominant As form in acuatic organisms including fish where Ab represents more than 95% of As total (84,85).
As compounds are absorbed by the gastrointestinal tract. Ingested inorganic As average biological life is about 10 hours and 50-80% is excreted in about three days, while methylated As has an average life of 30 hours. Ingested As may go through the placental barrier affecting the fetus, it is transported in the blood joined to the red cells and it is distributed throughout the body; once absorbed, it is oxidized and methylated in the liver to form monomethylarsonic acid and dimethylarsinic; this process may lead to the formation of dimethylated As metabolites. Most As is readily excreted in urine as MMA and DMA (5).
As has high affinity with the sulfhydryl groups, rich in keratin and tend to concentrate on the skin but it may be deposited also in bones, teeth, hair, and fingernails, mostly when the exposure is chronic (5). The evident signs of chronic As toxicity are skin changes and wart or callus formation on palms or soles, along interspersed hyperpigmentation areas on the face, neck, and back (86). Chronic exposure may produce neurotoxicity of the central and peripheral nervous system while the signs of As acute toxicity are mainly: vomit, diarrhea, cramps, salivation, fever, cardiovascular disorders, and it may lead to death (5). Arsenic is carcinogenic for group 1 humans and it is mainly related with lung, kidney, bladder, and skin cancer (28). Arsenic compounds affect human immune function. In environmentally exposed children, there is not correlation between total arsenic in urine and superoxide anion production and in adult smelter workers, higher levels of urinary arsenic correlated with increased lipid peroxidation and lower vitamin E levels in blood (5).
According to researches conducted in Brazil, China, Mediterranean Sea and Taiwan, among analyzed toxic metals As concentrations were the highest (54, 56, 59, 57). The highest and lowest observed were 2.61 ± 1.47 µg As/ g wet weight for T. thynuss and 0.39± 0.25 µg As/ g wet weight for L. griseus in Mediterranean Sea and the Pearl River delta region (China) (56,59). Chen et al., reported that As concentrations in fish muscles varied depending on the capture place which are widely influenced by human activities (54). As concentration reported in Brazil and Taiwan were similar: 0.600 ± 0.200 25 µg As/g wet weight for C. crysos and 0.683 ± 0.175 for M. cyprinoids (54, 57) .
PTWI for inorganic As previous recommendations of 2.1 µg As/Kg of body weight determined by JECFA have been withdrawn because they are considered without protective effect (87).
DISCUSSION
This paper develops general and important aspects of pollution of Hg, Cd, Pb and As in some fisheries resources of commercial importance, give an overview of each metal, their natural and anthropogenic sources, cycles and speciations, toxicity and health effects, state regulations and recommendations for tolerable intake. It is important to note that the information shown here provide an overview of the conditions that determine the concentration of Hg, Cd, Pb and As in marine ecosystems.
In the global context, studies on trace elements contents in fish and their health risks are related to factors like the characteristics of the fish, according to Denton et al. Chen et al. in their researches, Hg contents in fish muscular tissue and liver tissue are clearly dependent on trophic levels, observing higher mercury levels in largest, most enduring, and predatory fish (46, 54). However, not only the species, the size, and the age of the fish are the determining factors for a higher metal content, as Ploetz et al. point out in their S. cavalla study which did not find any correlation between the size of the fish and its muscle cadmium content (11) and Chen et al. did not observe differences in the As concentrations according to the species of the fish (54). Other characteristics are also determinant for biota metalscontent, as pointed out by Velusamy et al in their Mumbay Bay (India) findings, with higher trace metal accumulation in demersal fish followed by neritic and pelagic fish (88). Additionally, aquatic ecosystem environmental conditions like the level of contamination, increase metal concentrations due to fish susceptibility to toxic substances present in water, as stated by Thiyagarajan et al., on their studies in different zones on the southwest coast of India related to high populations and industrial effluents as the main metal contamination sources in coastal waters (55).
Some studies have given importance to the effects of the season on metal concentration in fish, making comparisons between the winter and summer seasons. Saei-Dehkordi and Fallah refer in their Persian Gulf studies conditions as water temperature, fish dietary factors, and the growth as determinants in the fluctuation of metal concentrations. Researchers have found higher mercury, arsenic, cadmium, and lead levels in fish during the winter in comparison to the summer; these significant differences may be attributed to the precipitation of waste caused by rain (58, 72). In other findings, Marrugo et al. reported contradictions in higher Hg values during the dry season; however, it is mainly associated to the increase of contaminated sources (mining activities) during this season (60).
Trace elements content varies depending on the zone, environmental conditions, the contamination level of the fishing area, and the characteristics of the fish (size, endurance, and diet) constitute relevant factors, being some fish more prone to accumulating higher concentrations of these metals in the muscle. Data taken from researches conducted in the Gulf of Mexico, Iran, Brazil, Australia, China, India, Egypt, western and central Pacific Ocean, Mediterranean Sea and Taiwan, where Hg, Cd, Pb and As contents in Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, Thunnus> and Megalops fish genera were determined, (Table 1), allows determining safe consumption limits according to Hg, Cd, Pb and As content in fish, using the method proposed by Ikem and Egievor (89), when comparing these data with allowed tolerable intake according to Codex Alimentarius and JECFA international regulation and using the fish consumption recommendation used by FDA and U.S. EPA for 8-12 ounce weekly consumption (23); for instance: If an average 70 Kg adult is considered with an ingest of 360 g/week of C. melampygus fish in Queensland (Australia) with a mercury content of 0.804 µg Hg /g wet weight (Table 1) which corresponds to a value of 4.134 µg Hg/kg of body weight per week (0.804 µg Hg/g wet weight x 360 g/70 kg), this consumption would represent possible risks given it is above the 4 µg Hg/ kg of body weight PTWI determined for humans by CODEX and JECFA (63). Taking into account that MeHg contribution to total mercury in fish generally corresponds to between 80% and 100%, using the same calculations for a 360 g/week consumption of C. melampygus of the Queensland study, it would represent a weekly ingest of 3.307 µg MeHg/kg of body weight, exceeding the 1.6 µg/kg of body weight PTWI for humans determined by CODEX and JECFA (63, 64). Through this method it is possible to estimate the risk of fish consumption according to metal content in the Cd case data collected in the US, Trinidad, Brazil, China, Iran, India, and Taiwan for S. Commerson, E. Coioides, C. crysos, L. griseus, L. stellatus and M. cyprinoids species; none of them represented a health risk because they did not exceed the 25 µg Cd/ Kg of body weight PTMI determined by CODEX and JECFA (63, 73). Currently, there are no availed CODEX and JECFA criteria to determine safe Pb and As ingests (63, 81, 87).
Usually in highly contaminated aquatic habitats the concentrations of metals in the muscle of the fish, particularly mercury, exceed the permissible limits for human consumption and involve serious health threats (46, 49), however some investigators as Kehrig et al. Havelková et al. and Farkas et al. have shown that metals concentration in fish muscle can also be found in areas with low or absent sources of pollution (90-92).
Levels of Hg, Cd, Pb and As in water bodies determine significantly the content of these elements in fish tissues (55, 93), therefore it's not enough with only know characteristics such as species, age and size of the fish, but also take into account these metal releases from natural and anthropogenic sources into marine ecosystems. Although exist reports of the metals concentration in several oceans from the world, more studies about their distribution in the water bodies are required to understand the metals behavior in marine currents, allow the development of models for measuring the relationship between emission sites and reception regions (94, 95).
The limitations of this review lie in the few information about the marine currents effects in the distribution of trace elements across water bodies, which makes it difficult assessing the relationship between emission source and receptor region and how this affect the distribution, accumulation and concentration of metals in marine ecosystems, on the other hand it is difficult to conclude one specific cause that determines the content of toxic metals in fish species, since this is not only a single factor but the whole of them. A future revision requires more research that clearly concludes on the ecotoxicology, cycles and speciation of some metals and their effects on the human body, as well as relations between water bodies and the distribution of trace elements.
CONCLUSION
The Hg, Cd, Pb and As content in fish tissue are determine by several aspects that includes both fish characteristics and metal behavior in the marine ecosystem, by which is important take in count each of these, in any study of this field. Also in important note that metal content in fish represents a public health risk in populations with frequent fish consumption, due to the toxicity it may represent to the different human body systems. Among these metals, mercury outstands due to its high levels of concentration in the muscular tissue in some fish species and the capacity to form more toxic compounds, as well as its high availability in aquatic ecosystems due to constant industrial use, being, particularly, gold mining in the South American case. Some of the species studied in the present paper are migratory fish, that is an important bioindicator of the increase contamination from the research areas. Studies submitted in this review, may be used as the base for future comparisons with Hg, Cd, Pb and As concentration values in different fish studies for the Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, Thunnus and Megalops genera in order to be able to determine consumption recommendations and warnings.
ACKNOWLEDGEMENTS
We would like to thank Antioquia Governor's Office Royalty General System, the financing entity, and Universidad de Antioquia.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
1. Patterson J. Introduction - Comparative dietary risk: Balance the risk and benefits of fish consumption. Comments Toxicol. 2002;8(4-6):337-43. [ Links ]
2. Kadar I, Koncz J, Fekete S. Experimental study of Cd, Hg, Mo, Pb and Semovement in soil-plant-animal systems. krmiva. 2001;43(3):185-90. [ Links ]
3. Burger J, Gochfeld M. A framework and information needs for the management of the risks from consumption of self-caught fish. Environ Res. 2006;101(2):275-85. [ Links ]
4. Kojadinovic J, Potier M, Le Corre M, Cosson RP, Bustamante P. Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ Pollut. 2007;146(2):548-66. [ Links ]
5. Tokar EJ, Boyd WA, Freedman JH, Waalkes MP. Toxic effects of metals. Casarett and Doull's Toxicology. 8th ed. McGraw-HilL; 2015. p. 933-80. [ Links ]
6. Zhang L, Wong MH. Environmental mercury contamination in China: Sources and impacts. Environ Int. 2007;33(1):108-21. [ Links ]
7. Agency for Toxic Substance and Disease Registry. Toxicological Profile for Lead. Atlanta: U.S. Department of Health and Humans Services, Public Health Service, Centres for Diseases Control; 2007. [ Links ]
8. Agency for Toxic Substance and Disease Registry. Toxicological Profile for Arsenic. Atlanta: U.S. Department of Health and Humans Services, Public Health Service, Centres for Diseases Control; 2007. [ Links ]
9. Henley RW, Berger BR. Nature's refineries - Metals and metalloids in arc volcanoes. Earth-Science Rev. 2013;125:146-70. [ Links ]
10. He ZL, Yang XE, Stoffella PJ. Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol. 2005;19(2-3):125-40. [ Links ]
11. Ploetz DM, Fitts BE, Rice TM. Differential accumulation of heavy metals in muscle and liver of a marine fish, (King Mackerel, Scomberomorus cavalla Cuvier) from the Northern Gulf of Mexico, USA. Bull Environ Contam Toxicol. 2007;78(2):124-7. [ Links ]
12. Chandra Sekhar K, Chary NS, Kamala CT, Suman Raj DS, Sreenivasa Rao A. Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environ Int. 2004;29(7):1001-8. [ Links ]
13. Cheng-xiu LU, Jie-min C. Speciation of Heavy Metals in the Sediments from Different Eutrophic Lakes of China. Procedia Eng. 2011;18:318-23. [ Links ]
14. Pempkowiak J, Sikora A, Biernacka E. Speciation of heavy metals in marine sediments vs their bioaccumulation by mussels. Chemosphere. 1999;39(2):313-21. [ Links ]
15. Zhang L, Shi Z, Zhang J, Jiang Z, Wang F, Huang X. Spatial and seasonal characteristics of dissolved heavy metals in the east and west Guangdong coastal waters, South China. Mar Pollut Bull. Elsevier Ltd; 2015;95(1):419-26. [ Links ]
16. Nfon E, Cousins IT, Järvinen O, Mukherjee AB, Verta M, Broman D. Trophodynamics of mercury and other trace elements in a pelagic food chain from the Baltic Sea. Sci Total Environ. Elsevier B.V.; 2009;407(24):6267-74. [ Links ]
17. Olmedo P, Pla A, Hernández a. F, Barbier F, Ayouni L, Gil F. Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environ Int. Elsevier Ltd; 2013;59:63-72. [ Links ]
18. Qiu Y-W. Bioaccumulation of heavy metals both in wild and mariculture food chains in Daya Bay, South China. Estuar Coast Shelf Sci. Elsevier Ltd; 2015;163:7-14. [ Links ]
19. Gray JS. Biomagnification in marine systems: the perspective of an ecologist. Mar Pollut Bull. 2002;45:46-52. [ Links ]
20. Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. [ Links ]
21. Agency for Toxic Substance and Disease Registry. Substance Priority List. Department of Health and Humans Services, Public Health Service,Centres for Diseases Control, Atlanta, GA.; 2013. [ Links ]
22. Castro-González MI, Méndez-Armenta M. Heavy metals: Implications associated to fish consumption. Environ Toxicol Pharmacol. 2008;26(3):263-71. [ Links ]
23. United States Environmental Protection Agency. Fish: what pregnant women and parents should know [Internet]. 2014 (cited 2014 Sep 6). Available from: http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm393070.htm [ Links ]
24. Ceccatelli S, Daré E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact. 2010;188(2):301-8. [ Links ]
25. Carocci A, Rovito N, Sinicropi MS, Genchi G. Mercury toxicity and neurodegenerative effects. Reviews of Environmental Contamination and Toxicology. 2014. p. 1-18. [ Links ]
26. Zhang W-L, Du Y, Zhai M-M, Shang Q. Cadmium exposure and its health effects: A 19-year follow-up study of a polluted area in China. Sci Total Environ. 2014;470-471:224-8. [ Links ]
27. Méndez-Armenta M, Ríos C. Cadmium neurotoxicity. Environ Toxicol Pharmacol. 2007;23(3):350-8. [ Links ]
28. International Agency for Research in Cancer. Monographs on the evaluation on carcinogenics risks to humans [Internet]. 2014 (cited 2014 Sep 11). Available from: http://monographs.iarc.fr/ENG/Classification/ [ Links ]
29. Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie. 2006;88(11):1549-59. [ Links ]
30. Gwaltney-Brant SM, Haschek WM, Rousseaux CG, Wallig MA. Chapter 41 - Heavy Metals. Haschek and Rousseaux's Handbook of Toxicologic Pathology (Third Edition). Boston: Academic Press; 2013. p. 1315-47. [ Links ]
31. Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006/02/28 ed. Instituto de Fisiologia, Universidad Autónoma de Puebla, Pue, México.; 2006;12(3):57-65. [ Links ]
32. Abernathy CO, Thomas DJ, Calderon RL. Health effects and risk assessment of arsenic. J Nutr. 2003;133((5 Suppl 1)):1536s - 8s. [ Links ]
33. Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133(1):1-16. [ Links ]
34. McCurry J. Japan remembers Minamata. Lancet. England; 2006. p. 99-100. [ Links ]
35. Inaba T, Kobayashi E, Suwazono Y, Uetani M, Oishi M, Nakagawa H, et al. Estimation of cumulative cadmium intake causing Itai–itai disease. Toxicol Lett. 2005;159(2):192-201. [ Links ]
36. De Jesus IS, da Silva Medeiros RL, Cestari MM, de Almeida Bezerra M, de Mello Affonso PRA. Analysis of Metal Contamination and Bioindicator Potential of Predatory Fish Species Along Contas River Basin in Northeastern Brazil. Bull Environ Contam Toxicol. 2014;1-6. [ Links ]
37. Olivero J, Johnson B. El lado gris de la minería del oro: la contaminación con mercurio en el norte de Colombia. Colombia: Universidad de Cartagena; 2002. [ Links ]
38. Bustamante C, Olivares F, Jimenez W. Calderos de mayor importancia en la pesca artesanal de Golfo de Urabá. Autoridad nacional de acuicultura y pesca; 2012. [ Links ]
39. SEPEC.
40. Ministerio de Ambiente Vivienda y Desarrollo territorial. Cuantificación de liberaciones antropogénicas de mercurio en colombia. República de Colombia; 2010. p. 0-82. [ Links ]
41. EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal. 2012;10:241. [ Links ]
42. Tan SW, Meiller JC, Mahaffey KR. The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol. 2009;39(3):228-69. [ Links ]
43. Debes F, Weihe P, Grandjean P. Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex. 2015 Jun; [ Links ]
44. Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E. Review Evidence on the Human Health Effects of Low-Level Methylmercury Exposure. Env Heal Perspect. 2012;120(6):799-806. [ Links ]
45. Enviromental Protection Agency (EPA). Mercury in Marine Life Database. U.S. Office of Wetlands, Oceans, and Wathersheds. Washington, DC; 2003. [ Links ]
46. Denton GRW, Burdon-Jones C. Trace metals in fish from the Great Barrier Reef. Mar Pollut Bull. 1986;17:201-9. [ Links ]
47. El-Moselhy KM, Othman AI, Abd El-Azem H, El-Metwally ME a. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt J Basic Appl Sci. Elsevier Ltd; 2014;1(2):1-9. [ Links ]
48. Ordiano-Flores A, Rosíles-Martínez R, Galván-Magaña F. Biomagnification of mercury and its antagonistic interaction with selenium in yellowfin tuna Thunnus albacares in the trophic web of Baja California Sur, Mexico. Ecotoxicol Environ Saf. 2012;86:182-7. [ Links ]
49. Chen CY, Lai CC, Chen KS, Hsu CC, Hung CC, Chen MH. Total and organic mercury concentrations in the muscles of Pacific albacore (Thunnus alalunga) and bigeye tuna (Thunnus obesus). Mar Pollut Bull. Elsevier Ltd; 2014;85(2):606-12. [ Links ]
50. Lima APS, Sarkis JES, Shihomatsu HM, Müller RCS. Mercury and selenium concentrations in fish samples from Cachoeira do PiriáMunicipality, ParáState, Brazil. Environ Res. 2005;97(3):236-44. [ Links ]
51. Dabeka R, McKenzie AD, Forsyth DS, Conacher HB. Survey of total mercury in some edible fish and shellfish species collected in Canada in 2002. Food Addit Contam. 2004;21(5):434-40. [ Links ]
52. Mendez E, Giudice H, Pereira A, Inocente G, Medina D. Total Mercury ContentâFish Weight Relationship in Swordfish (Xiphias gladius) Caught in the Southwest Atlantic Ocean. J Food Compos Anal. 2001;14(5):453-60. [ Links ]
53. Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Env Heal Perspect. 2003;111(4):604-8. [ Links ]
54. Chen YC, Chen CY, Hwang HJ, Chang WB, Yeh WJ, Chen MH. Comparison of the metal concentrations in muscle and liver tissues of fishes from the Erren River, Southwestern Taiwan, after the restoration in 2000. J Food Drug Anal. 2004;12(4):358-66. [ Links ]
55. Thiyagarajan D, Dhaneesh K V, Kumar TTA, Kumaresan S, Balasubramanian T. Metals in fish along the southeast coast of India. Bull Environ Contam Toxicol. 2012;88(4):582-8. [ Links ]
56. Leung HM, Leung AOW, Wang HS, Ma KK, Liang Y, Ho KC, et al. Assessment of heavy metals/metalloid (As, Pb, Cd, Ni, Zn, Cr, Cu, Mn) concentrations in edible fish species tissue in the Pearl River Delta (PRD), China. Mar Pollut Bull. 2014;78:235-45. [ Links ]
57. Medeiros RJ, dos Santos LMG, Freire AS, Santelli RE, Braga AMCB, Krauss TM, et al. Determination of inorganic trace elements in edible marine fish from Rio de Janeiro State, Brazil. Food Control. 2012;23(2):535-41. [ Links ]
58. Saei-Dehkordi SS, Fallah AA. Determination of copper, lead, cadmium and zinc content in commercially valuable fish species from the Persian Gulf using derivative potentiometric stripping analysis. Microchem J. 2011;98:156-62. [ Links ]
59. Storelli MM, Storelli MM, Storelli A, Storelli A, Marcotrigiano GO, Marcotrigiano GO. Accumulation of mercury, cadmium, lead and arsenic in sword sh and blue n tuna from the Mediterranean Sea: A comparative study. Mar Pollut Bull. 2005;50:1004-7. [ Links ]
60. Marrugo-Negrete J, Benitez LN, Olivero-Verbel J. Distribution of mercury in several environmental compartments in an aquatic ecosystem impacted by gold mining in northern Colombia. Arch Env Contam Toxicol. 2008;55(2):305-16. [ Links ]
61. Olivero-Verbel J, Caballero-Gallardo K, Marrugo Negrete J. Relationship between localization of gold mining areas and hair mercury levels in people from Bolivar, north of Colombia. Biol Trace Elem Res. 2011;144:118-32. [ Links ]
62. Olivero J, Johnson B, Arguello E. Human exposure to mercury in San Jorge river basin, Colombia (South America). Sci Total Environ. 2002;289:41-7. [ Links ]
63. Codex Alimentarius Commission. Joint FAO/WHO food standards programme Codex Committee on contaminants in foods sixth session [Internet]. 2012 (cited 2014 Jun 10). Available from: ftp://ftp.fao.org/codex/meetings/cccf/cccf6/cf06_INFe.pdf. [ Links ]
64. FAO/WHO Expert Commite on Food Additives. Methylmercury [Internet]. 2011 (cited 2014 Nov 20). Available from: http://apps.who.int/food-additives-contaminants-jecfa-database/ chemical.aspx?chemID=3083 [ Links ]
65. Agency for Toxic Substance and Disease Registry. Toxicological Profile for Cadmium. Atlanta: U.S. Department of Health and Humans Services, Public Health Service, Centres for Diseases Control; 2012. [ Links ]
66. Auger P a., Machu E, Gorgues T, Grima N, Waeles M. Comparative study of potential transfer of natural and anthropogenic cadmium to plankton communities in the North-West African upwelling. Sci Total Environ. Elsevier B.V.; 2015;505:870-88. [ Links ]
67. Horner TJ, Lee RBY, Henderson GM, Rickaby REM. Nonspecific uptake and homeostasis drive the oceanic cadmium cycle. Proc Natl Acad Sci U S A. 2013;110(7):2500-5. [ Links ]
68. Hendry KR, Rickaby REM, de Hoog JCM, Weston K, Rehkämper M. Cadmium and phosphate in coastal Antarctic seawater: Implications for Southern Ocean nutrient cycling. Mar Chem. Elsevier B.V.; 2008;112(3-4):149-57. [ Links ]
69. Houston MC. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, andmyocardial infraction. Altern Ther Heal Med. 13:128-33. [ Links ]
70. Kim K. Blood cadmium concentration and lipid profile in Korean adults. Environ Res. 2012;112:225-9. [ Links ]
71. Thatcher RW, Lester ML, McAlester R, Horts R. Effects of low levels of cadmium and lead on cognitive functions in children. Arch Environ Heal. 37:159-66. [ Links ]
72. Saei-Dehkordi SS, Fallah AA, Nematollahi A. Arsenic and mercury in commercially valuable fish species from the Persian Gulf: Influence of season and habitat. Food Chem Toxicol. 2010;48(10):2945-50. [ Links ]
73. FAO/WHO Expert Committe on Food Additives. Cadmium [Internet]. 2013 (cited 2014 Nov 20). Available from: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=1376 [ Links ]
74. Sepe A, Ciaralli L, Ciprotti M, Giordano R, Fumari E, Costantini S. Determination of cadmium, chromium, lead and vanadium in six fish species from the Adriatic Sea . Food Addit Contam. 2003;20:543-52. [ Links ]
75. Chakraborty P, Babu PVR, Sarma V V. A study of lead and cadmium speciation in some estuarine and coastal sediments. Chem Geol. Elsevier B.V.; 2012;294-295:217-25. [ Links ]
76. Woosley RJ, Millero FJ. Pitzer model for the speciation of lead chloride and carbonate complexes in natural waters. Mar Chem. Elsevier B.V.; 2013;149:1-7. [ Links ]
77. Heier LS, Lien IB, Strømseng AE, Ljønes M, Rosseland BO, Tollefsen KE, et al. Speciation of lead, copper, zinc and antimony in water draining a shooting range-Time dependant metal accumulation and biomarker responses in brown trout (Salmo trutta L.). Sci Total Environ. Elsevier B.V.; 2009;407(13):4047-55. [ Links ]
78. Papanikolaou CN, Hatzidaki GE, Belivanis S, Tzanakakis GN, Tsatsakis MA. Lead toxicity update, A brief review. Med Sci Monit. 2005;11:329-36. [ Links ]
79. Liu P, Wang C-N, Song X-Y, Wu Y-N. Dietary intake of lead and cadmium by children and adults - Result calculated from dietary recall and available lead/cadmium level in food in comparison to result from food duplicate diet method. Int J Hyg Environ Health. 2010;213(6):450-7. [ Links ]
80. Eisler R. Compendium of Trace Metals and Marine Biota. Compendium of Trace Metals and Marine Biota. 2010. [ Links ]
81. FAO/WHO Expert Committe on Food Additives. Lead [Internet]. 2011 (cited 2014 Nov 20). Available from: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=3511 [ Links ]
82. Matschullat J. Arsenic in the geosphere--a review. Sci Total Environ. 2000;249:297-312. [ Links ]
83. Marlborough SJ, Wilson VL. Arsenic speciation driving risk based corrective action. Sci Total Environ. Elsevier B.V.; 2015;520:253-9. [ Links ]
84. Ruttens a., Blanpain a. C, De Temmerman L, Waegeneers N. Arsenic speciation in food in Belgium. Part 1: Fish, molluscs and crustaceans. J Geochemical Explor. Elsevier B.V.; 2012;121:55-61. [ Links ]
85. Amlund H, Ingebrigtsen K, Hylland K, Ruus A, Eriksen DØ, Berntssen MHG. Disposition of arsenobetaine in two marine fish species following administration of a single oral dose of [14C] arsenobetaine. Comp Biochem Physiol - C Toxicol Pharmacol. 2006;143(2):171-8. [ Links ]
86. Kakkar P, Jaffery NF. Biological markers for metal toxicity. Environ Toxicol Pharmacol. 2005;19(2):335-49. [ Links ]
87. FAO/WHO Expert Commite on Food Aditives. Arsenic [Internet]. 2011 (cited 2014 Nov 20). Available from: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=1863 [ Links ]
88. Velusamy A, Satheesh Kumar P, Ram A, Chinnadurai S. Bioaccumulation of heavy metals in commercially important marine fishes from Mumbai Harbor, India. Mar Pollut Bull. 2014;81:218-24. [ Links ]
89. Ikem A, Egiebor NO. Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America). J Food Compos Anal. 2005;18(8):771-87. [ Links ]
90. Kehrig H a., Malm O, Moreira I. Mercury in a widely consumed fish Micropogonias furnieri (Demarest, 1823) from four main Brazilian estuaries. Sci Total Environ. 1998;213:263-71. [ Links ]
91. Havelková M, Dusek L, Némethová D, Poleszczuk G, Svobodová Z. Comparison of mercury distribution between liver and muscle - A biomonitoring of fish from lightly and heavily contaminated localities. Sensors. 2008;8(7):4095-109. [ Links ]
92. Farkas A, Salánki J, Specziár A. Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res. 2003;37(5):959- 64. [ Links ]
93. França S, Vinagre C, Caçador I, Cabral HN. Heavy metal concentrations in sediment, benthic invertebrates and fish in three salt marsh areas subjected to different pollution loads in the Tagus Estuary (Portugal). Mar Pollut Bull. 2005;50(9):998-1003. [ Links ]
94. Aparicio-González A, Duarte CM, Tovar-Sánchez A. Trace metals in deep ocean waters: A review. J Mar Syst. Elsevier B.V.; 2012;100-101:26-33. [ Links ]
95. Kocman D, Horvat M, Pirrone N, Cinnirella S. Contribution of contaminated sites to the global mercury budget. Environ Res. Elsevier; 2013;125:160-70. [ Links ]