Introduction
According to the World Health Organization (WHO)1, traditional medicine is an important instrument for pharmaceutical assistance, since approximately 70-90% of the world population uses herbal medicines as the only manner to access basic healthcare1,2 for both the prevention and control of diseases3.
In Brazil, 82% of citizens have consumed these kind of products4 for treatment of minor disorders and chronic illness5. In general, ethnobotanical knowledge underlies indication, dosage, and mode of administration of herbal medicines6. However, according to Rodrigues and Barnes7, this traditional framework is limited in the detection of adverse reactions, contraindications, toxicity, and other important aspects to establish the safety profile of natural substances.
Herbal medicines are associated with the development of serious adverse reactions8-10, which may cause life-threatening9, hospital admissions11, and death8,9,12. In addition, other factors may increase the risk of occurrence of adverse events, such as tampering, poor quality, lack of proper identification, contamination and inappropriate use13) as well as improper storage and handling14 and packaging15.
However, there is an underestimation of causal association between adverse effects and the use herbal medicines, since users do not inform health professionals about their administration2,14,16, and since they are not able to correlate unwanted clinical manifestation with intake17. Besides, the lack of awareness among professionals in requesting information about the consumption of herbal medicines during pharmacological anamnesis as well as the absence of a specific form to reporting adverse events related to medicinal plants and phytotherapic18 also hinder safety surveillance.
Therefore, more effective methods for recognition of negative clinical outcomes associated with use of herbal medicines are needed to strengthen actions of phytosurveillance in Brazil. In this setting, the present study aimed to perform the following: i) develop an instrument to report adverse events related to herbal medicines and ii) verify its ability to perform causality assessment and improve risk communication.
Material and methods
Study population and design
A quantitative methodological development research was carried out with health professionals who practiced in the northwest region of São Paulo State from March 2011 to March 2012 in order to develop an instrument to report problems of safety, therapeutic failure, and quality deviations of herbal medicines.
All primary healthcare professionals (physicians, pharmacists, nurses, dentists, and nutritionists) who agreed to participate in the study and signed an informed consent form were enrolled. Exclusion criteria comprised professionals who, after three attempts, did not respond to the invitation to participate in the study as well as those who returned unfilled forms in the second phase of the research. Therefore, it is an intentional sample applied to instruments developed to be pre-tested19.
Instrument development (form)
Generation of the initial sample of items
The form to report adverse events related to the use of herbal medicines was designed according to WHO guidelines20, which advocate the presence of several items to allow quality information, such as the following: 1) identification of patient (initials, demographic characteristics, medical history, and risk factors); 2) the suspected product (species, Latin and popular names, part used, method of preparation, manufacture, country of origin, batch, supplier, and validity); 3) mode of administration (dose and route); 4) indication and reason for use; 5) adverse event (clinical evolution, laboratory tests, onset and end of the event, seriousness, data of withdrawn, and rechallenge); 6) concomitant medications (prescription drugs and self-medication); and 7)demographic data of the reporter.
The instrument was designed in a semi-structured way with an essay and multiple choice questions21 in order to enable identification of problems related to safety, quality, and effectiveness of herbal medicines (Figure 1). It can be accessed here: http://www2.fcfar.unesp.br/Home/Alunos/naf/formulario-de-notificacao-de-reacoes-adversas-a-fitoterapicos-e-plantas-medicinais.pdf.
Qualitative assessment of the items
Qualitative assessment of technical terms applied to the development of the form. The items of the instrument were evaluated by two PhD docents of School of Pharmaceutical Sciences of Unesp. All fields were considered applicable for the population of the study. There was not the need to reformulate any items.
Data collection
Assessment of appropriateness of the instrument to report adverse events validation was performed in three phases.
During the first phase a pilot study was conducted with undergraduate students taking a pharmacy course at the School of Pharmaceutical Sciences in Unesp. A clinical case (case 1) of adverse reaction with likelihood of excellence (probability of occurrence classified as definite) was developed and distributed among volunteers.
Case 1 described a married woman (45 years old) who reported the use of eucalyptus leaves to treat nasal congestion. According to the patient, the leaves were collected in a field near her house. When she felt nasal congestion, she boiled an unmeasured quantity of leaves with water. The vapor produced was inhaled. After three weeks of treatment, she experienced abdominal pain, diarrhea, and moments of delirium. She also noted cyanosis in her extremities. After clinical assessment by a physician, she was informed to discontinue the use of the plant. Concomitant pharmacotherapy included acetylsalicylic acid 500mg once a day and captopril 25mg three times per day. A few weeks after discontinuing the herbal medicine, the patient recovered.
Moreover, samples of suspected plant were sent to undergraduate students in order to analyze them and complete the instrument.
The second and third phases of the study were performed according to the methodology developed by Naranjo et al. (1981) (22. Therefore, a test-retest was carried out.
The second phase comprised the distribution of Case 1 for primary care health professionals as well as the samples of the suspect plant. Subjects should analyze the case and the samples to report them in the instrument.
A week later (third phase), a new clinical case (Case 2) was made available for health professionals. Case 2 described a single woman (50 years old) who reported feeling stress and insomnia. Her neighbor recommended Kava-kava to treat her anxiety and sleep difficulties. She prepared tea with leaves during several months or cooked the roots with coconut milk. She bought the plant from an herbalist. After three months, the patient noticed the occurrence of a cutaneous rash, red eyes, and yellow nails and fingers. She ingests alcoholic beverages during the weekends and has clinical history of hepatitis. To recover from hangovers, she uses acetaminophen (750 mg). Her physician recommended discontinuation of the plant, and after ten days, she completely recovered.
Herbal samples were also distributed among volunteers in the third phase. They analyzed the material and filled out the instrument. Herbal samples provided were not necessarily responsible for the occurrence of the observed event. We opted for this strategy to assess whether the volunteers would perform the analysis of the material, allowing their identification.
Clinical cases were elaborated based on scientific articles23-26 available in the databases PubMed and Lilacs. Since each case had its own specified age and sex of patients as well as the reason and mode of use of herbal medicines, clinical history, and polypharmacy, several gold standard answers were adopted: satisfactory fulfillment of essential, necessary, and recommended fields regarding information related to identification of patients and plants; drugs used concomitantly and clinical history; and data of the reporter.
Data Analysis
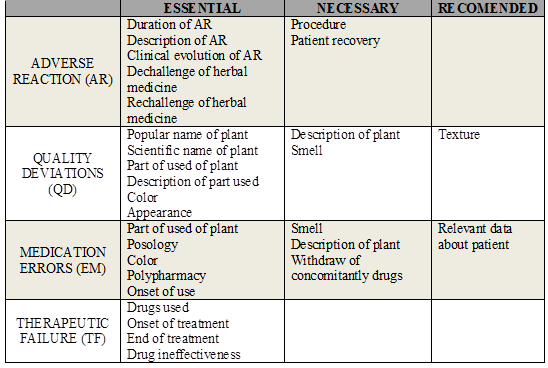
Content analysis of the instrument with both clinical cases was performed, comparing the items filled by health professionals with a gold-standard1. OPS (2011)1) classified the information to be reported according to the degree of relevance: essential, necessary and recommended.
Then, content analysis considered the fill of the correspondent items in the form, according to all adverse events identified in clinical cases and relevance of information, as proposal by OPS. The agreement of volunteers to complete the information in the fields considered essential, necessary, and recommended to be reported was demonstrated using coefficient kappa.
Essential items are those which allow identifying the suspicious plant, the adverse event, and the patient. The completion of these fields enables the analysis of causality, since it promotes assessment of temporal association (development of adverse event and the intake of the drug); pharmacological plausibility (if the mechanism of action of the active principle may explain the occurrence of the adverse event); the exclusion of alternative causes (other situations that could explain the development of the observed event, such as drug interactions, concomitant drugs, clinical condition of the patient, among others); and verifying that the event has been described for the plant used.
Necessary items complement causality analysis and allow the assessment of the clinical course of the adverse events (outcomes, seriousness, dechallenge, and rechallenge). The lack of necessary information in the form does not hinder causality assessment, since follow up makes the collection of missing data possible in order to complete the evaluation.
Recommended items collaborate with classification of adverse events in adverse reaction, quality deviations, therapeutic failure, or medication errors.
Accuracy and integrity of reports were analyzed according to the proportions of the within-rater reliability in completing the items classified as essential, necessary, and recommended to be reported. Thus, the kappa coefficient was calculated. It was stipulated that 90 %, 80 %, and 50 % of the essential, recommended, and necessary items should be filled, respectively. Week agreement was observed when 0.21 < k < 0.40, moderate agreement was observed when 0.41 < k < 0.60, good agreement was observed when 0.61 < k < 0.80 and very good agreement was observed when 0.81 < k < 1.0.
Results
The pilot study was carried out with 20 students. Most volunteers (n=18) filled the adverse events identified in the correspondent item in form correctly. These findings show the terminology used to develop the instrument is adequate to report adverse reactions and quality deviations. However, due to lack of data regarding medication errors and therapeutic failure in the reports, these items were included to contribute with reporting of any kind of problems related to herbal medicines.
A hundred and seven health professionals were invited to participated of phase 2, of which 57 (53.3%) returned both forms completed (48 pharmacists; five nutritionists; two nurses; a physician; and a dentist). According to demographic characteristics, most volunteers were women (n=49) and non-elderly people (mean 30.5 years old; minimum 21 and maximum 60 years old).
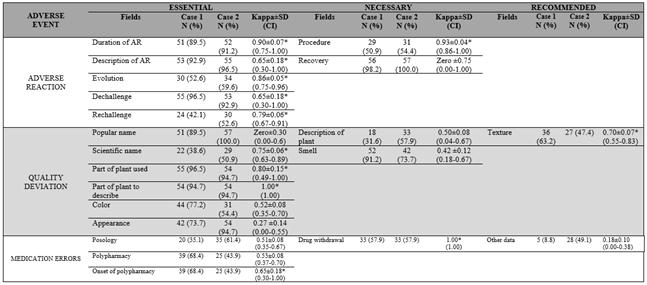
Data show that 12 out of 21 fields of instrument had moderate (n=7) and very good agreement (n=5): nine of them were essential, two were necessary, and one was recommended, respectively (Table 1).
Table 1 Classification of form´s fields according to degree of relevance and indication of reporting.
In general, the proposed instrument was able to identify adverse reaction, quality deviation, and medication errors, since among the 14 essential fields (Table 1), 12 of them showed a high degree of within-rater reliability (k > 0.61) (Table 2). Five items contribute for identification of adverse reaction; five promote identification of quality deviation; and the other three provide recognition of medication errors.
Among five necessary fields (Table 1), two (Table 2) showed moderate within-rater reliability (0.41 ≤ k ≤ 0.61), which was related to the identification of quality deviations. Considering the two fields recommended to be filled in the form (Table 1), one was related to medication errors (Table 2), which had poor agreement (k < 0.41).
Items associated with causality assessment that enable definitive adverse reaction imputations (duration, description, evolution of reaction, and dechallenge and rechallenge of plant) showed a high degree of within-rater reliability (k > 0.61) in both clinical cases (Table 2).
Discussion
Signal generation in pharmacovigilance depends on the quantity and quality of adverse drug reports submitted to sanitary agencies27. In Brazil, after development of electronic reporting of adverse events (NOTIVISA), in 2008, no reports of problems related to the use of herbal medicines was received by the National Surveillance Agency (ANVISA)8. Our findings suggest the form develop is an appropriate instrument to report adverse events arising from herbal medicines.
In Africa, although herbal medicine pharmacovigilance programs covered suspected adverse reactions in 14 (87.5%) countries, medication errors in 5 (31.2%) countries, falsification and adulteration in 2 (each 12.5%) countries, and drug interactions in 1 (6.3%) country, particular attention to the development of surveillance of natural products is required28.
Underreporting18 and poor quality of information hinders causality association 29,30. Therefore, specific tools are needed to contribute for analysis of safety, quality, and effectiveness of these products18.
The proposed instrument met this need, since it was effective in detecting adverse events related to herbal medicines. According to Barnes (2003)18, a specific instrument appropriately designed to report and to assess the causality of adverse events arising from herbal medicines may be a strategy to decrease underreporting in phytosurveillance
Furthermore, the instrument contributed for recognition of adverse reactions with high likelihood (definite and probable). Maximum degree of imputation depends on dechallenge and rechallenge of natural substance with recurrent of undesired effects31. These fields, as well as those related to “description” and “evolution of reaction”, were satisfactorily filled in both cases reported by health professionals. Therefore, there is evidence that our form contributes for the improvement of the quality of information reported, which is essential to causality assessment of adverse reaction.
Recovery field is not considered an essential item, since reports per se do not allow follow up of each case32, clinical outcomes are unknown during the detection of a suspicion of adverse event. Therefore, although this field has been poorly filled, this is not a limitation of the proposed instrument, since the outcomes arising from adverse events may be collected during the follow up of the cases.
The improvement of quality of information by proposed form also contributed to the detection of medication errors, mainly, prescription and administration. For example, cases of administration errors can be minimized by filling in fields "part of the plant used"; this enables verification whether the correct part of herbal medicine was used and "mode of use" favors the analysis of suitable extraction method and dosing schedule.
The fields of “polypharmacy”; “onset of concomitant drugs", and “suspension of use" allows identifying prescriptions errors, such as indication and drug interactions. Skalli et al. (2012)33) stated that reports of drug interactions involving herbal medicines and allopathic drugs are very important to delineate the safety profile of these products. The poor concordance obtained in the field “relevant data” (where volunteers would inform morbidity and co-morbidity, drug allergies, and liver or kidney failure) make the analysis of therapeutic failure and toxicity difficult, since they may be developed by multifactorial conditions, such as genetic polymorphisms, low dose, overdose, tolerance, quality deviations, adulterations, among others. Correctly completing this field should improve the recognition of the contribution of the plant in the development of adverse event.
Low within-rater reliability might be explained considering that, in the first case, there was no clinical relevant information worthy of note, since the patient was healthy. However, it was expected that professionals could signal the absence of alternative causes that could contribute to the occurrence of the medication errors verified.
Finally, the instrument identifies quality deviations in a regularly way, especially regarding to tampering and contaminating herbal medicine samples. This finding can be justified based on the subjectivity associated to items "smell", "color", "appearance", and "texture". However, satisfactory filled fields "scientific name" may minimize bias related to description of organoleptic characteristics provided by health professionals, since it is possible to compare it with a description provided by official literature; however, this may increase quality deviations15,34. Moreover, the scientific name of herbal medicine is important for the standardization and harmonization of terminology within an international framework17 in order to increase the effectiveness of post-marketing in regulation and supervision of natural products available in the market.
To improve the documentation of adverse event reports, mainly of quality deviations, the development of educational interventions for health professionals is recommended. These methods have proven effective to increase knowledge, attitudes, and skills regarding the spontaneous reporting of adverse drug events35,36. Therefore, we suggest adaptation of the approach and their content for monitoring of herbal medicines in order to fundamentally standardize the analysis of the organoleptic parameters of the products and to highlight the importance to notify problems of safety, quality, and effectiveness related to the use of natural substances.
The awareness of health professionals and users of herbal medicines began in 2007, when the Brazilian Information Center on Psychotropic Drugs (Cebrid) launched the newsletter called PANFLAVI, which is release three times each year37.
A pharmacovigilance system of herbal medicines also started in the same year by the publication of a notification form of adverse reactions related to herbal medicines (RAMP) by the institution. Therefore, the instrument form developed in this study will allow identification of other potential adverse events associated with the use of these products, such as handling and packaging14,15, improper storage and identification15, serious adverse reactions38, and medication errors13.
This instrument is suitable for identification of adverse events related to herbal medicines, since it improves upon the previously available forms by ANVISA and CEBRID and expands the possibility of identification of events other than adverse reaction. The present form differs from reports of allopathic drugs, since it provides fields regarding organoleptic characteristics (smell, color, texture, appearance) and mode of use. Thus, it is intended to improve adverse event reporting related to herbal medicines and to contribute for appropriate use by expanding the usage information.
Limitations of the study
Our findings should be interpreted with caution, because of the following limitations. Due to clinical cases, this study only included adverse reactions to herbal medicines; sessions relating to phytotherapics have not been assessed. Also, the form does not meet the criteria for notification of ready mixes of herbal medicines due to the difficulty of identifying the components of formulations. Furthermore, we use a non-probabilistic sample due to the lack of access of a complete list of health professionals in the region of the study. Then, findings should not be generalized.
Conclusions
The instrument developed in this study is suitable for identification of adverse events related to herbal medicines. Thus, it contributes to risk communication in phytosurveillance, since it allows voluntary reporting and causality assessment of the events identified. The instrument is also able to satisfactorily identify safety issues with a high degree of imputation (definitive) and quality deviations problems. The present form differs from reports of allopathic drugs, since it provides fields regarding organoleptic characteristics (smell, color, texture, appearance) and mode of use. Thus, it is intended to improve adverse event reporting related to herbal medicines and to contribute for appropriate use by including usage information.
Acknowledgements
We would like to thank the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), and São Paulo Research Foundation (FAPESP) for the master’s and Ph.D. scholarships that they provided (case 99999.014301/2013-00) as well as their sponsorship of research (FAPESP 2013/12681-2
Authors’ contributions
Patricia de Carvalho Mastroianni and Luis Vitor da Silva Sacramento contributed to conceptualization, methodology, supervision, validation and writing (review and editing). Marília Amaral Costa and Fabiana Rossi Varallo contributed to data collection, analysis, validation, visualization and writing (original draft preparation)