Introduction
The Mahogany tree (Swietenia macrophylla King), which can usually reach 40 to 60 meters, belongs to the Meliaceae family and has a tropical distribution found in Central and South America, in Western India, in Malaysia and in the central and southern China 1,2. This plant is used in Asia for the treatment of various diseases based on their antimicrobial effects, anti-inflammatory, antioxidant, anti-mutagenic, anti-cancer, anti-tumor and anti-diabetic activities 2. Its seeds have many uses, such as in the traditional treatment of hypertension, diabetes and to relieve pain in Malaysia, to treat leishmaniosis and to induce abortion in Bolivia, as well as for the treatment of diabetes, hypertension and malaria in Indonesia. In addition, its bark is used as an astringent for wounds and occasionally for sun tanning 2-6.
Phytochemical studies of the acetone-soluble, ethanolic, and methanolic extracts have shown that limonoids and its derivatives are the main constituents of S. macrophylla. Limonoids derived from tetracyclic triterpenes eufol like (H-20β) or tirucalol (H-20α) by a series of oxidative changes, interspersed with molecular rearrangements 7. Most of the limonoids that have been evaluated were obtained from seed extracts 8. In the leaves of this species carbohydrates, free or combined flavonoids, sterols and/or triterpenes, hydrolyzable and condensed tannins, essential oils as Himacaleno-γ, germacrene-D, germacrene-A , diene-1,4-Cadina, decahexanoic acid and ethyl hexadecanoate have been reported. Also limonoids, pragmaline type, from the leaf dicloromethanolic extract, called "Swietephragmine" HJ 1-3 and "Swietemacrophine" were described. From its bark three compounds with antioxidant activity were reported, namely, phenylpropanoid substituted catechin (Swietemacrophilanine), catechin, and epicatechin; all of them with antioxidant activity 9-11.
With the leaf ethanolic extract of S. macrophylla, promising results were obtained in an in vitro study by inhibiting myotoxic phospholipases A2 (PLA2) and Crotoxin from the venom of South American pit vipers (Bothrops asper, Bothrops atrox and C. durissus cumanensis). In addition, this extract did not show toxicity to cultured muscle cells. In this same study, it was reported that polar fractions derived from the leaf ethanolic extract were rich in phenolic compounds 12,13, which are suggested to be involved in the inhibitory activity of phospholipase A2 as previously described 14. In a subsequent study were a bioassay-guided fractionation was carried out with the ethanolic leaf extract of S. macrophylla, the activity of several fractions against PLA2s from B. atrox, B. asper and C. durissus cumanensis in vitro and in vivo were evaluated. One of the most polar fraction, named " Sm13-16,23", exhibited an inhibition of the mentioned PLA2 higher than 80% 15. These kind of results are promising in the search for adjuvants for the treatment of snakebite accidents. However further studies are required in order to establish the toxicity of promising samples before recommending its use.
In the present study, based on previous studies that have reported that some polyphenols have in vitro and in vivo inhibitory activities of the venoms of Elapids and Vipers snakes, 16-19, and considering the promising inhibitory results obtained previously with fraction “Sm13-16,23" derived from S. macrophylla15, the aim of this study was to determine the acute oral toxicity of this fraction. Although there are publications about the toxicity of this plant, they have been assessed only in seed extracts, possibly because it is most used in traditional medicine. However, the leaves toxicity is unknown, which led to the purpose of this study in order to complement previous results.
Materials and methods
Plant material
Fraction "Sm13-16,23" was obtained by a bioassay-guided fractionation of the leaf ethanolic extract of S. macrophylla15, which was previously collected on campus of the National University of Colombia, located in Medellin (Voucher sample, TL-103). Briefly, air-dried and milled leaves were extracted by percolation with ethanol. The resultant ethanolic extract was fractionated by a silica-gel open column eluted with a gradient of organic solvents, which resulted in 22 fractions, from which fractions 13 to 16 were pooled based on its chromatographic profile and its bioactivity. The combined fractions F13 to F16 were fractionated by silica gel as well, resulting in 30 subfractions, from which subfraction 23 was the most active inhibiting the PLA2. This subfraction was labelled as “Sm13-16,23”. In further characterization, studies 2-3 compounds were observed in analytical HPLC (Shimadzu), from which polyphenolic compounds were detected by the presence of typical signals for hydroxyl and aromatic rings through IR and UV spectral data 15.
This sample was kept frozen until bioassays. For biological analysis it was diluted in ethanol and placed in a vortex for three minutes, then brought to ultrasound for seven minutes, filtered using a 0.2 µm membrane and dried in SpeedVac (Vacufuge plus, Eppendorf). Afterwards it was diluted in a sterile saline solution (SSF) 0.9% (Baxter, Colombia), vortexed for three minutes, and brought to the ultrasound seven minutes again before administration to animals.
Experimental animals
This study was approved by the Ethics Committee for Animal Experimentation (CEEA) of the “Universidad de Antioquia” (Act Nro.87 of January 30, 2014). Conventional Swiss Webster mice between 20 to 25 grams body weight were used. The animals belonged to Animal House Department of “Universidad de Antioquia” Research Place, remained with artificial light, 12 hours photoperiod, temperature of 22 ± 1 °C and relative humidity of 60 ± 4%. Before each experiment a five days acclimatization period was made.
Acute oral toxicity
To determine the acute oral toxicity of the extract, the parameters described in the guide No. 423 of 2001 of the Organization for Economic Cooperation and Development (OECD) were followed 20. Five mice as treatment group and five mice as control group were used. Two assays were made, the first at a dose 2000 mg/kg and the second with a lower dose of 300 mg/kg. The treatment group received 0.2 mL SSF with the diluted “Sm13-16,23” fraction, which was administered by gavage. The control group received only 0.2 mL of SSF. Prior to gavage, mice were maintained in a four hours fast, which lasts up to an hour after the treatment administration. All animals were observed continuously for the first hour, then daily for 14 days. Daily monitoring of behavior, weight and medical condition of each mouse was performed. The consciousness, body condition, the presence of piloerection, lethargy, stupor or other alterations in the organic system, was evaluated. Finally, on day 14 an overdose of CO2 euthanasia was performed.
Histopathological analysis
Necropsy of all animals was performed and macroscopic findings were detailed. Stomach, small intestine, colon, spleen, liver, lungs, kidneys, heart, brain, and gastrocnemius muscle were sampled. Tissue samples were fixed in 10% buffered formalin, embedded in paraffin and cut into 4μm thick. All tissues were stained with Hematoxylin Eosin (H-E) 21. For histopathological analysis, sections were evaluated under an optical microscope Leica DMLB (Meyer Instruments, Houston, TX, USA) and a semi-quantitative assessment of lesions were given (Table 1). All samples were processed and analyzed in the laboratory of Animal Pathology of Universidad de Antioquia.
Statistical analysis
To evaluate differences between groups, nonparametric Mann-Whitney test (this test was chosen because then was very low to comply a normal distribution) and repeated measure ANOVA was used. The value of p <0.05 was considered statistically significant. Analyses were performed in the SPSS software (IBM, Statistic Version 20).
Results
During clinical monitoring of animals, no changes in clinical or behavioral parameters of the animals or death were observed at any doses tested. In Figure 1 and Figure 2 it is shown that at doses of 2000 mg/kg the fraction induced statistically significant variations in animal weight (p = 0.034), this was not observed at doses of 300mg/kg (p = 0.208). At necropsy, no macroscopic alterations were found in the organs in any of the administered doses.
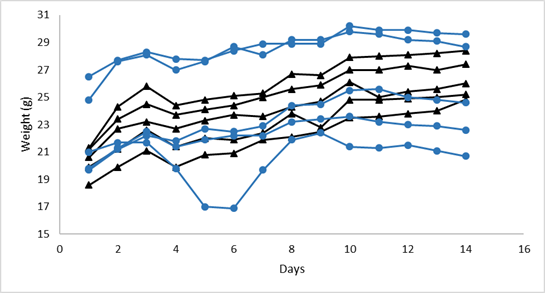
Figure 1 Body weight (g) monitored in the control animals (() and treated animals (•) at doses of 2000 mg/kg (n=10).

Figure 2 Body weight (g) monitored in the control animals (() and treated animals (•) at doses of 300 mg/kg (n=10).
Histopathological analysis
The dose of 2000 mg/kg significantly increases the karyomegaly (p = 0.004) and the binucleation (p = 0.007) of hepatocytes in all treated animals (n = 5) compared to controls (Figure 3). At the same dose induced mild kidney tubular necrosis in one animal and slight vacuolar degeneration; however, these lesions were not statistically significant different between groups (p = 0.138 and p = 0.056, respectively). In other organs lesions were not observed. In animals in the control group they showed no injuries. Table 2 shows the lesion on each organ and extent of histopathologic findings described.
Table 2: Histopathological findings of treated animals with Sm13-26 fraction at doses to 2000 mg/kg (n=5)
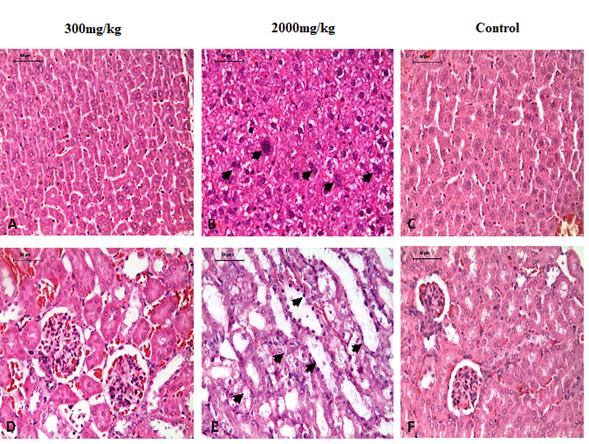
Figure 3 Detailed liver (A and B) and kidney (D and E) between the evaluated doses of Sm13-26,23 fraction. A and D: 300 mg/kg, B and E: 2000 mg/kg, C and F: Controls. In figure B arrows show karyomegaly and binucleation at 2000 mg/kg. In figure E arrows show the tubular necrosis and nephrosis. Hematoxylin-Eosin stain (H-E, 400x).
At dose of 300 mg/kg no histopathological lesions were observed in any organ of the treated animals. The control animals showed no lesion.
Discussion
Although some plants have been used in folk medicine for many years is necessary evaluated the safety of them in controlled studies 22. Different plants had been report with inhibitory activity of snake venom (23, 24). However, the acute toxicity had been evaluate for only some of them. The aqueous extract of the roots of Aristolochia indica protected the animals injected of Russell's viper venom and did not evidenced toxicity in animals at lower doses, but in high dose cause liver and kidney damage 25. Renealmia alpiniaextracts provided protection against lethal effects of B.asper venom and had no toxic effects in mice 26. The same way Peristrophe bivalvis extracts with antivenom potential to Naja kaouthia and Trimeresurus albolabris, did not show toxic signs in rats 27.
Although they have been used
in folk medicine for many years, few well controlled
studies in humans and animals have been conducted,
and a need exists for additional safety studies in animals
and humans. In addition, more detailed chemical analy-
ses are required so that relationships between chemical
composition and physiological and pharmacological
actions can be establish
Although they have been used
in folk medicine for many years, few well controlled
studies in humans and animals have been conducted,
and a need exists for additional safety studies in animals
and humans. In addition, more detailed chemical analy-
ses are required so that relationships between chemical
composition and physiological and pharmacological
actions can be establish
Although they have been used
in folk medicine for many years, few well controlled
studies in humans and animals have been conducted,
and a need exists for additional safety studies in animals
and humans. In addition, more detailed chemical analy-
ses are required so that relationships between chemical
composition and physiological and pharmacological
actions can be establish.
The toxicity of the seed extract of various species of Swietenia were previously described, in a recent study of acute oral toxicity of the seed extract from Swietenia mahagoni in a murine model, lesions suggestive of organ toxicity at doses of 25, 200 2000, 5000 mg/kg were not observed 28. In another study which the acute oral toxicity of seed extract from Swietenia macrophylla in rats at doses of 2000 mg/kg was evaluated, the results showed that seeds did not produce significant differences in weight, food and water intake, biochemical and hematological parameters, or macroscopic and histopathological changes in the organs of the treated animals compared with controls 6.
In this study fraction “Sm13-16,23" derived from the ethanolic leaf extract of S. macrophylla did not produce death or clinical signs of acute oral toxicity in mice at doses of 2000 mg/kg and 300 mg/kg. Nevertheless, the dose of 2000 mg/kg induced variations in the weight of the animals without changes in food consumption, but no significant lesions were observed at necropsy. In the histopathological findings it increased karyomegaly and binucleation of hepatocytes when compared to the controls, and nephrosis and mild renal tubular necrosis in some of the treated animals was observed with this higher dose.
It is to consider that an increase in polyploidy of the hepatocytes can occur with age, because the number of chromosomes may increase from 16 to 32, therefore, the karyomegaly, anisokaryosis and policaria or multinucleation are incidental findings in rodents 29. However, the increase in the multinucleation that was evidenced at the dosage of 2000 mg/kg, can be related to regenerative or degenerative conditions of the liver, while the karyomegaly or megalocytosis could be associated to the xenotoxicty caused by non-necrotizing compounds that hinder regeneration 29. The vacuolar nephrosis and renal tubular necrosis lesions are suggestive of nephrotoxicity.
In ruminants and occasionally in horses, ingestion of large amounts of polyphenols, such as tannins can cause hydrothorax, ascites, perirrenal edema, ulceration of the alimentary tract, acute tubular necrosis and death. Although in less serious injuries, adjacent groups of renal tubules with varying degrees of degeneration appear 30, which is according with our findings. In this respect, some of the metabolites responsible for the toxicity of plants are gallotannins, which are hydrolyzed to gallic acid, pyrogallol and tannic acid 30. As stated previously, the preliminary characterization of fraction Sm13-26,23 pointed to the presence of phenolic compounds in this fraction, which is relevant considering the supporting literature for this type of compounds 15. However, in this study, we did not observe the injuries provoked by tannins poisoning in ruminants and horses, suggesting that fraction Sm13-26,23 has not a high content of tannins.
In contrast, this fraction has other polyphenols 31 as discussed above, which could have induced cellular changes. Nevertheless, in other study from leaves aqueous extract of Swetenia were identified nine phenolic acids and 18 flavonoids with antioxidant ability on cell culture, suggesting a cytoprotecting activity 32.
In the same way a recent study of Swietenia macrophylla leaves extracts, catechin was the most abundant constituent in some fractions with antioxidant and rich polyphenols 31. In other study about the toxicity of green catechins studies, showed no toxic effects in animals. Additionally, demonstrated reduced body weight in 5% 33, similar to found in our study. The results of this study are in accordance with the established by the OECD 20, fraction Sm13-26,23 did not produce toxicity at doses of 300mg/kg, indicating that the risk of acute oral toxicity is low.
Conclusions
The Fraction Sm13-16, 23 of Swietenia macrophylla promising in the search for inhibitors of snake venom, did not produce toxicity or lesions at doses of 300mg/kg, indicating that the acute oral toxicity risk is low.
Acknowledgments
We are thankful to our colleagues in the Serpentarium, Ophidism/Scorpionism Program, and Animal Pathology Lab from the College of Agricultural Sciences. This work was possible to the funding obtained by the “Universidad de Antioquia” CODI Project 8700-170 and Sostenibilidad (2014-2015) UdeA.
Authors’ contributions
A.H. performed acute toxicity assays, histopathological analysis and wrote the manuscript; B.R performed histopathological analysis and help with manuscript preparation; AP: Obtained the fraction of Swietenia macrophylla and help with manuscript preparation; TL: coordinated the obtained fraction of S. macrophylla and help with manuscript preparation and VN: Coordinated project, help with acute toxicity assays, and help with manuscript preparation.















