Introduction
Benign prostatic hypertrophy (BPH), the non-malignant enlargement of the prostate due to cellular hyperplasia, is a very common urological problem present in at least one third of men older than 50 years that leads to lower urinary tract symptoms (LUTS), diminished quality of life and increased risk of urological cancer 1. LUTS secondary to BPH are related to smooth-muscle tension in the prostate tissue, a process that is mediated by alpha-1-adrenergic receptors. Therefore, alpha-1-receptor blockade is the mainstay pharmacological therapy for the treatment of moderate-to-severe BPH 2. Tamsulosin and silodosin are uroselective agents for this disorder due to their high relative affinity for the alpha-1a receptor subtype, but alpha-1 antagonists like doxazosin, terazosin, alfuzosin and prazosin are equally effective 2. Other type of agents used for this disorder, depending on the severity and associated complications include the use of 5-alpha-reductase inhibitors and phosphodiesterase-5 enzyme inhibitors 3.
Products from natural sources are very commonly used for LUTS secondary to BPH. Serenoa repens fruit (“Saw palmetto”), Hypoxis rooperi roots (“South African star grass”), Pygeum africanum bark (“African plum tree”), Urtica dioica roots (“Stinging nettle”), Secale cereale pollen (“Rye”) and Cucurbita pepo seeds (“Pumpkin”) are among the most popular species consumed for this purpose. Suggested active compounds include: phytosterols, fatty acids, lectins, flavonoids, plant oils and polysaccharides. Possible effects ascribed are of varying types: antiandrogenic, antiestrogenic, antiedematous, anti-inflammatory, with mechanisms related to inhibition of 5-alpha-reductase, blockage of alpha-receptors, inhibition of prostatic cell proliferation, inhibition of prostaglandins and strengthening of detrusor muscle. However, more research is required to determine their efficacy in LUTS of BPH 2,4,5.
Achyrocline bogotensis (Kunth) [N.V. “Vira Vira”, Compositae] is a Colombian medicinal plant used traditionally in decoction for inflammatory and infectious disorders of the skin, respiratory tract, urinary tract and prostate (LUTS), among others 6-8. Flavone and cyclobutane dimer compounds are among its metabolites, which have shown in vitro antiviral, antineoplastic and immunomodulatory actions 9-12. Achyrocline species subjected to chemical and biological studies include A. satureioides13-16, A. alata17-19, A. tomentosa, (20, 21) and A. flaccida (22, 23). The profile of their biological activities include anti-inflammatory, anti-infective and antioxidant effects related, at least in part, to their polyphenol components. A. satureioides and flavonoid compounds isolated from this species have shown relaxant effects on the smooth muscle of guinea pig corpus cavernosum 24.
In spite of popular use of A. bogotensis for LUTS, there are no studies regarding its effect on smooth muscle sensitive to alpha receptor stimulation. This work aimed to assess the smooth muscle relaxant effect of A. bogotensis extract related to its alpha-1 antiadrenergic properties using aortic rings isolated from Wistar rats in a tissue bath.
Materials and methods
Extraction and Fractionation
The aerial parts from plant material used in this study were collected from “Páramo La Esperanza”, Sutamarchán region (Boyacá, Colombia). A voucher specimen (Col 522900) was deposited in the Colombian National Herbarium of the Institute of Natural Sciences, Bogotá, (Botanist Edgar Linares). The Stems and leaves of A. bogotensis were dried in a forced air oven at 40°C and milled. The powder obtained (1128 g) was macerated with 96% ethanol, filtered and concentrated under reduced pressure. The ethanol extract obtained (217.5 g; 19.3% p/p) was partitioned with distilled water, butanol and dichloromethane to yield three respective fractions. The ethanol extract and the obtained fractions were subjected to phytochemical screening according to the methods described by Sanabria (1983). For the in vitro experiments, the extract and fractions were dissolved in dimethylsulfoxide (DMSO, 0.01%).
Experimental protocol
The study was performed in a tissue bath containing isolated aortic rings obtained from male Wistar rats provided by Animalarium of Pharmacy Department, School of Sciences, Universidad Nacional de Colombia. The animals were maintained at a controlled temperature (22 ± 1°C), with 12 h light/dark cycles and water and food consumption ad libitum, except for the test day when rats were fasted for12 hours. Rats (250-400 g) were anesthetized with ether and sacrificed by cervical dislocation. The descending thoracic aorta was dissected and placed in an oxygenated Krebs solution with the following composition (in mM): NaCl, 118.0; KCl, 4,75; CaCl2, 1,8; MgSO4, 1,2; KH2PO4, 1,2; NaHCO3, 25; glucose, 11 and ascorbic acid 0,1. Rings of thoracic aorta (4-5 mm in length) were carefully excised and submerged in bath organ chambers containing 10 ml of Krebs solution of bathing medium maintained at 37°C and bubbled with 95% O2 and 5% CO2 gas mixture (pH 7.4). The rings were mounted by means of two parallel L-shaped stainless-steel holders inserted into the lumen. One holder served as an anchor while the other was connected to a force-displacement transducer (Harvard UF1) that measured the isometric contractile force and recorded it via a computer system (Videograph CODAS CI). A basal tension of 2 g was applied. Each preparation was allowed to equilibrate for 60-90 min in Krebs solution prior to the initiation of experimental procedures, and during this period the incubation media was changed every 15 min.
In the screening assay, after equilibration, the aortic rings incubated in Krebs solution were exposed to the ethanol extract of A. bogotensis (10 µg/mL), prazosin (1 µM, as reference standard) and (DMSO, 0.1%, as control). Fifteen minutes later, the rings were stimulated with cumulative increasing doses of the alpha-1 agonist phenylephrine (PE, from 0.1 nM to 50 uM) or the calcium channel voltage dependent activator KCl (from 10 µM to 140 mM). Then, according to these results, the capacity of the A. bogotensis ethanol extract to reduce the PE pressor effect (pD2' value) was quantified by assaying 1, 5 and 10 µg/mL concentrations.
In another set of experiments, the A. bogotensis relaxant potency was determined in aortic rings stimulated with KCl (80 mM) or PE (1 µM) until the contractile response reached a steady tension. After that, cumulative concentrations of the ethanol extract (0.1 µg/mL - 0.1 mg/mL), were added to isolated aortic rings pre-contracted with PE (0.1 µM) or KCl (80 mM).
To explore the possible participation of nitric oxide (NO), L-NAME (100 µM, NO synthase inhibitor) was administered to aortic rings before adding cumulative concentrations of PE to isolated aortic rings in the presence of the extract (10 µg/mL).
To assess the response obtained due to extract fractioning, the aqueous, butanol and dichloromethane fractions (10 µg/mL) were assayed in rings stimulated cumulatively with PE.
All provisions concerning the protection of animals for experiments stipulated by Resolution 008430/1993 by the Ministerio de la Protección Social of Colombia were applied. This work applied experimental protocols endorsed by the Ethics Committee of the Faculty of Sciences of the National University of Colombia (Act 07-2016).
Data analysis
The response of the aortic rings is expressed as a percentage of the maximal plateau contraction (Emax) induced by KCl or PE. Concentration-response curves were analyzed to give the logarithm of KCl or PE concentrations producing a 50% of Emax (EC50: effective concentration 50) by sigmoidal curve-fitting analysis. In the same way, concentration-response curves to give the logarithm of the extract concentration required to relax isolated aortic rings previously stimulated with PE or KCl to 50% were obtained (IC50). Values of pEC50 and pIC50 are expressed as -logCE50 and -logCE50, respectively. pD2´ corresponds to -log of the extract concentration that reduces the agonist effect to 50% of Emax and was obtained by scathcard plot analysis 26,27.
All results are expressed as means ± the standard error of the mean (SEM). Differences in aorta concentration-response curves were analyzed by one way analysis of variance (ANOVA) followed by Dunnett post hoc tests, with a criterion set for statistical significance at p<0.05. Excel ® and GraphPad Prism ® software was used for data analysis.
Results
Control rings stimulated cumulatively with PE and KCl attained concentration - response contractions at a plateau (Emax) of 2401 ± 328 and 2207 ± 156 mg, respectively. Prazosin (1 µM) and A. bogotensis ethanol extract (10 µg/mL) notably reduced the contraction values induced by PE on Emax, CE50 and pEC50, (Table 1, Figure 1), and their inhibitory effect in rings contracted with KCl is shown in Table 2 and Figure 2.
Table 1 % Emax , pEC50, and EC50 values with fiducial limits generated by PE (from 0.1 nM to 50 uM) in the absence (control) and presence of A. bogotensis ethanol extract (10 µg/mL) or prazosin (1 µM) in isolated aortic rings from Wistar rats.
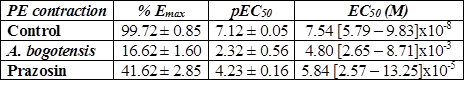
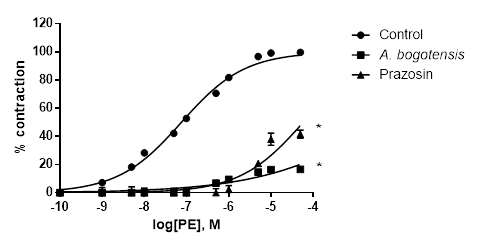
Figure 1 Percentage of contraction of isolated aortic rings from Wistar rats stimulated with cumulatively increasing concentrations of PE in absence (control) and presence of A. bogotensis ethanol extract (10 µg/mL) or prazosin (1 µM). Each point represents the mean ± S.E.M. n ≥ 8, (*p<0.05 vs. control).
Table 2 % Emax , pEC50, and EC50 values with fiducial limits generated by KCl (from 10 µM to 140 mM) in the absence (control) and presence of A. bogotensis ethanol extract (10 µg/mL) or prazosin (1 µM) in isolated aortic rings from Wistar rats.
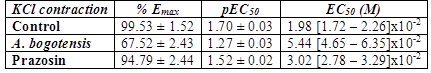
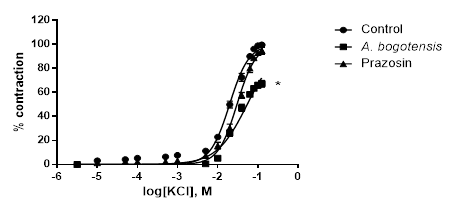
Figure 2 Percentage of contraction of isolated aortic rings from Wistar rats stimulated with cumulatively increasing concentrations of KCl in the absence (control) and presence of A. bogotensis ethanol extract (10 µg/mL) or prazosin (1 µM). Each point represents the mean ± S.E.M. n ≥ 8, (*p<0.05 vs. control).
A. bogotensis ethanol extract showed a high capacity for reducing the PE pressor response in a concentration dependent manner (1,5 and 10 µg/mL), giving a pD´2 value of 5.51 ± 1.70, (Figure 3).
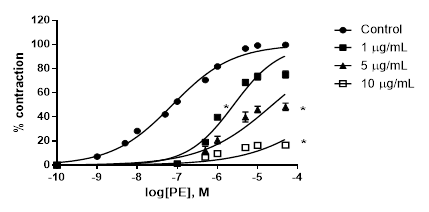
Figure 3 Percentage of contraction of isolated aortic rings from Wistar rats stimulated with cumulatively increasing concentrations of PE in the absence (control) and presence of A. bogotensis ethanol extract (1, 5 and10 µg/mL). Each point represents the mean ± S.E.M. n ≥ 8, (*p<0.05 vs. control).
A. bogotensis ethanol extract showed complete efficacy in relaxing rings previously precontracted with PE. The relaxant efficacy and potency of A. bogotensis extract against rings previously contracted with KCl were notably lower (Table 3, Figure 4).
Table 3 % Relaxation, pIC50, and IC50 values with fiducial limits generated by cumulative addition of A. bogotensis ethanol extract (1 - 100 µg/mL) in isolated rat aortic rings previously contracted with PE (1 µM) or KCl (80 mM).
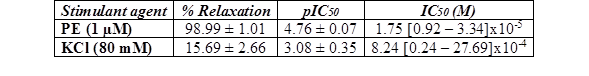
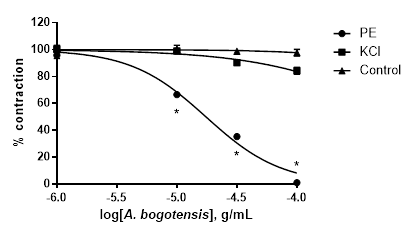
Figure 4 Effect of cumulative addition of A. bogotensis ethanol extract (1 - 100 µg/mL) in isolated aortic rings from Wistar rats previously contracted with PE (1 µM) or KCl (80 mM), (*p<0.05 versus control: DMSO: 0.1%).
L-NAME significantly reduced the inhibitory effect of A. bogotensis extract in rings stimulated with PE. Aqueous, butanol and dichloromethane fractions (10 µg/mL) gave inhibitory responses lower than that obtained with the ethanol extract (Table 4, Fig. 5).
Table 4 % Emax , pEC50, and EC50 values with fiducial limits generated by PE (from 0.1 nM to 50 uM) in the absence (control) and presence of A. bogotensis ethanol extract (10 µg/mL) plus L-NAME (100 µM) and the A. bogotensis fractions: aqueous, butanol and dichloromethane, in isolated aortic rings from Wistar rats.
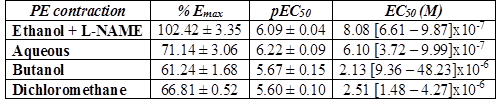
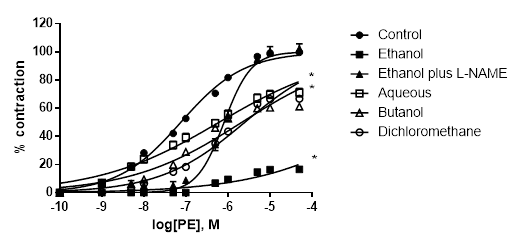
Figure 5 Percentage of contraction of isolated aortic rings from Wistar rats stimulated with cumulatively increasing concentrations of PE in the absence (control) and presence of A. bogotensis ethanol extract (10 µg/mL) plus L-NAME (100 µM), or in the presence of the A. bogotensis fractions: aqueous, butanol and dichloromethane. Each point represents the mean ± S.E.M. n ≥ 8, (*p<0.05 vs. control).
Phytochemical screening of the A. bogotensis ethanol extract showed significant presence of flavonoid metabolites according to positive results obtained in the Shinoda test, revealed with reactive NP-PEG. Triterpene and steroid metabolites were also present according to TLC using the Liebermann-Burchard test 25. Alkaloids and saponins were not detected whereas coumarin, tannin and anthraquinone metabolites were present in trace amounts.
Flash column chromatography studies were carried out using glass column with silica gel 60 (0.063-0.200 mm) as stationary phase. The mobile phase used were mix of CH2Cl2/MeOH in increasing polarity. Size-exclusion chromatography were carried out using glass column with sephadex LH-20 (25-100 mm), as stationary phase. The effluent from the columns was collected into test tubes, fractions with the same Rf (thin layer chromatography) were combined, concentrated and dried under reduced pressure. In thin layer chromatography, 0.25 mm thick pre-fabricated aluminum sheets were coated with silica gel with UV254 fluorescent indicator.
Discussion
These results show that the ethanol extract from the aerial parts of A. bogotensis displays a smooth muscle relaxant effect against PE whereas its effect against KCl is lower. This suggest that A. bogotensis displays some kind of direct or indirect alpha-1 inhibitory properties while it has less effective inhibitory actions on calcium voltage dependent channels. Because A. bogotensis could be exerting modulatory effects in conditions where there is overexpression of alpha-1 mediating receptors, and keeping in mind that its effect is comparable to a synthetic compound like prazosin, it could be proposed that A. bogotensis would be useful in the treatment of LUTS related to BHP. Until now, possible antiadrenergic properties of A. bogotensis have not been established.
The activity of A. bogotensis seems to be dependent, at least in part, on the presence of NO, because L-NAME, an inhibitor of NO synthesis, significantly reduces the inhibitory effect on isolated aortic rings stimulated with PE. In addition, given that the fractions obtained from the ethanol extract gave inhibitory responses lower than those obtained with it, it is possible to propose that more than one active metabolite is present in A. bogotensis. If that was not the case, fractionation of the extract should lead to increased inhibitory responses. Because there was a strong presence of flavonoid compounds found in the phytochemical screening, these kind of metabolites could be the main compounds responsible for the antiadrenergic properties of A. bogotensis.
Previous works have led to the isolation of t 3,5-dihydroxy-6,7,8-trimethoxy flavone from A. bogotensis, a compound that possess in vitro antineoplasic activity against several tumorigenic cell lines (9, 12, 28). This flavone could also play a pivotal role in the smooth muscle relaxant effects of A. bogotensis. It fulfills the criteria proposed for displaying relaxant effects according to structure-activity relationships studied for flavonoid compounds, particularly due to hydroxyl substitution of the B phenolic ring and the absence of these substitutions on the A phenolic ring (29, 30). This conformation would belong to the group of flavone compounds with the 2-phenyl-1,4-benzopyrone backbone.
As for several other flavones, this compound could improve endothelial function by increasing the bioavailability of NO by enhancing its synthesis and/or decreasing its superoxide-mediated breakdown. Therefore, it could help to protect against the development and progression of cardiovascular disease 31. Antioxidant actions would be at the root of its biological activity but other mechanisms could also play an important role (32, 33). Thus, the resulting alpha-1 inhibitory properties of. A. bogotensis would be attributable only in part to the presence of nitric oxide, and/or to its antioxidant profile. Additionally, other active compounds could play a role, including those of non-flavonoid nature, such as cyclobutane dimers (achyrodimers), among others 10.
The rat isolated aortic preparation is a suitable model for screening alpha 1-adrenoceptor antagonists 34 and hence, for assessing new agents potentially useful for the treatment of LUTS related to BPH. Tamsulosin, for example, an alpha-1a subtype selective antagonist, show greater relaxant potency in isolated rat aortic rings than in isolated rat vas deferens, an experimental model also routinely used to assess this kind of receptor 35. It is interesting, as found in the results of this work, that A. bogotensis displays comparable activity to that seen and described with the reference agent, prazosin 34.
Given that the smooth muscle of the urinary tract is rich in alpha-1a subtype receptors, selective agents like tamsulosin and silodosin are extensively used for LUTS due to BHP. However, adverse events related to alpha-1a interactions, such as floppy iris syndrome, are matters of concern 36. Therefore, non-selective alpha-1 antagonists remain a good alternative for the treatment of LUTS 37.
Future works with the bioactive principles of A. bogotensis will have to establish whether they elicit selective actions on alpha-1 receptor subtypes, or display additive or synergistic actions thus exerting a greater activity than the whole extract. In addition to functional studies, alpha-1 binding assays will be necessary 38. In any case, different mechanisms of alpha-1 interaction will have to be considered.
In conclusion, these results suggest that an extract from the aerial parts of the plant species A. bogotensis elicits smooth muscle relaxant effects related to the alpha-1 antiadrenergic mechanism. This response is partially NO dependent and seems to be due to interactions among active metabolites in the extract, which would be of flavonoid and/or terpenoid nature. These results give support to the traditional use of this species for LUTS due to BPH.














