INTRODUCTION
Hypertension, in spite of the advances in its detection and treatment, remains as a major risk for stroke, myocardial infarction, vascular disease and chronic kidney disease 1. Latin America and the Caribbean have some of the highest estimates of hypertension prevalence 2 and efforts to increase the awareness and control of this disorder are especially needed in this area 3.
Being called the “silent killer” in view the common absence of symptoms, hypertension constitutes a challenge for managing the adherence of patients to pharmacological treatment, as the adverse effect profile of several antihypertensive agents has to be confronted 4. Although “increasing adherence may have a greater effect on health than any improvement in specific medical treatments” 5, therapeutic non-compliance related to adverse events from current drugs and their insufficient efficacy to prevent complications related to hypertension in several cases are reasons that justify the search for new antihypertensive strategies.
In spite of the current use of combinatorial chemistry techniques as methods of optimising structures, natural products persist as the main source of potential innovative structures as bases for new drugs. Hypertension, a chronic disorder with high impact in public health remains a key target in the search for innovative antihypertensive agents 6.
The control of blood pressure maybe not be sufficient to effectively prevent the risk of cardiovascular disorders related to hypertension.
In fact, there seem to be differences between antihypertensive agents and antihypertensive groups used to reduce this risk. Such would be the case, for example, between angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers 7, between β-blockers and calcium channel blockers and renin-angiotensin system (RAS) inhibitors 8 and between thiazide diuretics and angiotensin converting enzyme (ACE) inhibitors in black and senior patients 9 inhibitors in black and senior patients. Therefore, when searching for new antihypertensive agents, analysis of the effect on the cardiovascular system, in addition to the levels attained of blood pressure in the experimental model applied, would afford more substantial information about the antihypertensive profile of a potential new drug.
Cardiovascular remodelling is a key factor that underlies the molecular, biochemical and cellular events leading to progressive dysfunction and lesions of the myocardium and vessels 10,11. Hypertension represents a pressure overload of the left ventricle wall, which, if not promptly managed, results in decreasing ventricular function and, consequently, in heart failure. At the same time, the increase in the thickness of small muscular resistance arteries, arterioles, and the microvasculature resulting from the remodelling of vessels in hypertension implies not only a maintained resistance due to excessive vasoconstriction, but a progressive lesion in the function of targets organs such as the brain, heart, kidney and retina. Therefore, antihypertensive agents that are able not only to reduce high blood levels but to ameliorate the progression of cardiovascular remodelling could represent an advantage in reducing the complication of hypertension 12.
The role of nitric oxide (NO) in the maintenance of the integrity of the endothelium and endocardium is well known, along with the fact that intima lesion of vessels affects the NO balance, leading to biochemical and morphological changes that trigger the inflammatory cascade of events implicated in the formation of thrombus and atheroma, as well as in the smooth muscle cell proliferation, apoptosis and fibrosis implicated in the remodelling process of hypertension 13,14,15. Therefore, NO cascade constitutes a key target for the management of hypertension and related cardiovascular disorders. Angiotensin converting enzyme inhibitors, statins and some beta blocking agents are among the drugs that favour NO production, reduce oxidative stress and ameliorate endothelial lesions 16,17.
Passiflora genus includes a variety of medicinal species among which hypertension is one of their ethnobotanical uses 18. P. edulis Sims extract, for example, decreases blood pressure in spontaneously hypertensive rats (SHRs) and contains metabolites like luteolin, luteolin-6-C-glucoside and gamma-aminobutyric acid (GABA) from the rind 19, phenolic compounds, ascorbic acid, carotenoids and flavonoids from the fruit pulp 20 and edulilic acid and anthocyanin from their leaves 21. Other Passiflora species studied are: P. nepalensis Walp 22, P. foetida Linn 23 and P. incarnate Linn 24.
In total, 90% of species from Passiflora are from America, with Colombia being one of the countries with the greatest richness 25,26, P quadrangularis L. (“N.V.” Badea) is common in this region and is traditionally used for the treatment of high blood pressure in this country, among other purposes 27. However, the antihypertensive properties of this species have not been previously studied in experimental models of hypertension.
This work shows the effect of the ethanol extract from the leaves of P. quadrangularis L. elicited in laboratory rats with hypertension induced by NO deficit, the ex vivo vascular response produced in animals previously treated with it, and the morphologic changes induced in the heart and aorta.
MATERIALS AND METHODS
Extraction and fractionation
Plant material from Passiflora quadrangularis L was collected from the region of Cartago (Valle del Cauca, Colombia, 1400 m. 22°C, Latitude 74°42’3.5244’’ - Longitude -75°55’11.337’’). Its identity was confirmed by comparison with a stored specimen (Code No. COL 06375, Herbarium of Faculty of Agricultural Sciences, Caldas University). The aerial part (approx. 12 kg of stems and leaves) was dried in an oven with circulating air at 40ºC for 48 hours. The dried material was ground and the resulting powder was macerated in EtOH for 72 h. Afterwards, it was filtered and concentrated in a rotary evaporator under reduced pressure until it was completely dry. The percentage yield was calculated respect to dry extract (18.80% w/w), the color and consistency of the extracts were noted (dark green and sticky semisolid mass). For in vivo assays, the extract was suspended in a vehicle resulting from a mixture of 10% propylene glycol, 10% glycerine and 2% polysorbate, with a stock concentration of 300 mg/mL. For in vitro assays, the extract was dissolved in dimethyl sulphoxide.
The alcohol extract was subjected to preliminary phytochemical analysis (28) for detection of the main secondary metabolites. The flash column chromatography studies were carried out using a column of 40cm x 3cm with silica gel MERCK® 60 (0,040-0,063mm) as stationary phase in ratio 50-60g of silica per gram of sample. The mobile phase used was hexane/CH2Cl2/MeOH increasing polarity. The effluent from the column was collected into test tubes; fractions with the same Rf (thin layer chromatography) were combined, concentrated and dried under reduced pressure.
In thin layer chromatography, 0.25 mm thick pre-fabricated POLYCHROM aluminum sheets were coated with silica gel with UV254 fluorescent indicator.
Experimental protocol
Female Wistar rats 43 were raised in colony cages and exposed to a 12 h dark/light cycle with controlled temperature and moisture (22ºC, 70%). They were fed a normal laboratory diet with free access to water and food. Cardiovascular experiments were carried out on rats aged 7-9 weeks and weighing 180-220 g. Female animals were used in this work, according to previous studies with Passiflora species and the need to include this genus in experimental models 29,30. The experimental procedure was approved by the institutional ethics committee (Act 14, October 2014, Faculty of Sciences, at the Universidad Nacional de Colombia). The animals were supplied by the Bioterium of the Pharmacy Department at the Universidad Nacional de Colombia.
Animals were previously acclimated during two weeks and then randomly assigned to the following treatment groups (n=6-8 per group): P. quadrangularis L. (three doses according to preliminary trials: 75, 150 and 300 mg/kg, p.o.), enalapril (as reference, 10 mg/kg, p.o), vehicle with L-NAME (as control group), and vehicle without L-NAME (blank group). L-NAME administration (10 mg/kg, i.p.) started in week 3 for all treatments except to the blank group. These treatments were administered every 48 h until the seventh week.
Measurement of blood pressure (IBP) and heart rate (HR)
Indirect blood pressure and heart rate were measured with a non-invasive method placing the base of the rat’s tail into the light of an ultrasound transducer (tail cuff device, PANLAB - LE 5002) capable of capturing the pulse signal and blood pressure. When external pressure is generated, the intensity of the pulse signal disappears due to the occlusion of blood vessels; the signal reappears when occlusion is reduced; this phase of the pulse corresponds to the systolic pressure 31. The transducer was plugged into a digital analogue recorder and software (LabTrax, DataTrax WPI).
The measurement was made by placing the experimental animal in a trap (after conditioning the animal in the trap for one more week) with a constant temperature of 29-32°C to produce the dilation in the tail of the animal, then the cuff is placed at the base of the tail. The systolic pressure records were made twice a week 32.
Aortic ring preparation
At the end of the treatment Wistar rats obtained from each experimental group (P. quadrangularis L., enalapril, blank and control) were anaesthetised with ether and sacrificed. The descending thoracic aorta was dissected and placed in a petri dish containing an oxygenated Krebs solution with the following composition (mM): NaCl 118.7; KCl 4.7; CaCl2 2.5; NaHC03 25.0; MgS04.7H20 1.2; glucose 11.0 and ascorbic acid 0.1. thoracic aorta rings (3-4 mm in length) were carefully excised and submerged in Allhin organ chambers containing 10 mL of Krebs solution bathing medium maintained at 37ºC, pH: 7.38 -7.42 and continuously gassed with a carbogen mixture of 95% O2 and 5% CO2. About 8-10 rings were obtained from each aorta 33.
Each ring was introduced inside an isolated organ bath containing 10 mL of Krebs solution maintained at 37°C and bubbled with carbogen. The ring was attached with two steel hooks, the inferior anchored to the bath and the upper connected to an isometric force transducer (Fort 10/WPI) coupled to an amplification and digital-analogue conversion system (Bridge 8/IsoDam, LabDataTrax, WPI) for signal analysis in the computer.
A basal tension of 2 g was applied to each preparation with a stabilisation period of 60-90 min, during which the Krebs solution was changed every 10-15 min. Once equilibrium was reached, the aortic rings incubated in Krebs solution were exposed to phenylephrine (FE, 10-6 M) until the contractile response reached a steady tension (approx. 40 minutes). Afterwards, ACh or SNP (from 10-10 to 10-4 M) was added cumulatively every 30 s in aliquots of 0.5 log units of concentration 34.
Histopathology of heart and aorta
The heart was dissected out and then weighed. Heart weight-to-body weight ratio (H/BW, mg/g index) was calculated 35. Excised heart and aorta samples were cleared of blood and immediately fixed in 10% formalin. 5 mm-thick tissue sections from heart and aorta were prepared from processed paraffin-embedded samples. Heart and aorta sections were stained with Harris haematoxylin and Masson’s trichrome stains and examined under a light microscope for evidence of hypertrophy and fibrotic changes 36. Measures of relations obtained from thickness of left ventricle and aorta wall versus its inner diameter (µm) were performed (W/D, wall thickness-to-diameter ratio) 37.
Solutions
The following drugs, salts and solutions were used: Enalapril (Enalapril 5mg Genfar®), L-NAME, phenylephrine (FE), acetylcholine (ACh), sodium nitroprusside (SNP), propylene glycol, polysorbate, glycerine, potassium chloride, magnesium sulphate, potassium hydrogen phosphate, L-ascorbic acid, sodium chloride, calcium chloride, sodium bicarbonate, and glucose (Sigma®).
Statistical and data analysis
All of the results are expressed as mean ± standard error mean (S.E.M.). Analysis of variance was performed followed by Dunett’s test to identify groups responsible for significant differences against control (p<0.05). Dose-response curves of isolated aortic rings were analysed by a sigmoid curve-fitting analysis to give the negative log of the concentration of ACh or SNP producing a 50% in the maximal relaxant response (pEC50). One - way ANOVA analysis was applied to histopathological results. Excel ®, GraphPad-Prism ® and OpenStat programs were used for data analysis.
RESULTS
Phytochemical analysis
Preliminary phytochemical analysis of alcohol extract of Passiflora quadrangularis L. leaves, revealed the presence of alkaloids (1.5%), flavonoids (14%) and saponins (80%) approximately. Flash column chromatography studies were carried out using glass column with silica gel MERCK ® 60 (0.040-0.063 mm) as stationary phase in ratio 20-30 g of silica per gram of sample. The mobile phase used were mix of Hexane-AcOEt or CH2Cl2/MeOH in increasing polarity. Size-exclusion chromatography were carried out using glass column with sephadex LH-20 FLUKA (25-100 mm), as stationary phase in ratio 100 g of sephadex per gram of sample. The mobile phase used were Hexane/CH2Cl2/MeOH (1:2:3) or H2O/CH2Cl2/MeOH (1:2:3). The effluent from the columns was collected into test tubes, fractions with the same Rf (thin layer chromatography) were combined, concentrated and dried under reduced pressure. In thin layer chromatography 0.25 mm thick pre-fabricated POLYCHROM aluminum sheets were coated with silica gel with UV254 fluorescent indicator.
Effects of P. quadrangularis L. on systolic arterial pressure and heart rate
The mean systolic arterial pressure value at basal conditions (week 1) ranged from 106 to 110 mmHg (n=47) without any significant differences between groups. The increase in arterial pressure induced by L-NAME was significant from week 6, reaching values of 150 mmHg. All doses of P. quadrangularis L were able to maintain pressure values below 121 mmHg for the seven weeks of treatment. The effect of the reference drug enalapril was similar to that of P. quadrangularis L and to the group of rats not exposed to L-NAME (blank group) (Figure 1). The basal heart rate value was 429±28 bpm and did not show any significant changes during the treatment for any of the groups.
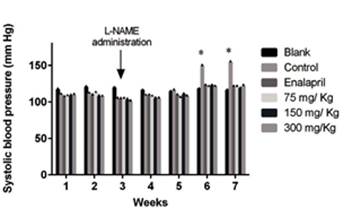
Figure 1 Values of systolic blood pressure in Wistar rats treated with: (1) L-NAME (10 mg/kg, i.p.) plus EtOH extract of P. quadrangularis L (75, 150 and 300 mg/kg/day, p.o.), (2) L-NAME plus enalapril (reference drug, 10 mg/kg/day, p.o.), (3) L-NAME plus vehicle (control; 10% propylene glycol, 10% glycerine and 2% polysorbate, 0.1 mL/100 g, p.o.) and (4) vehicle without L-NAME (blank). Results are expressed as means ± S.E.M. *p<0.05, with respect to the control group.
In vitro aortic ring studies
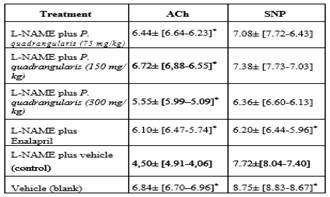
Stimulation of aortic rings with FE (10-6 M) resulted in a sustained contraction of 1962 ± 40 mg. The magnitude of this contraction was similar in all treatment groups. The cumulative addition of ACh (10-10 - 10 -4 M) in isolated aorta rings previously exposed to L-NAME and treated with P. quadrangularis L significantly displaced the curve to the left compared to the control group in a concentration-dependent manner (Figure 2). Enalapril also displaced the curve to the left. The cumulative addition of SNP (10-10 - 10-4 M) in isolated aorta rings treated with P. quadrangularis L. (all doses) and enalapril significantly displaced the curve to the right compared to the control (Figure 3). Therefore, P. quadrangularis L. increased the relaxant potency induced by ACh. Table 1 shows pEC50 values of ACh and SNP in the presence and absence of P. quadrangularis L or enalapril.
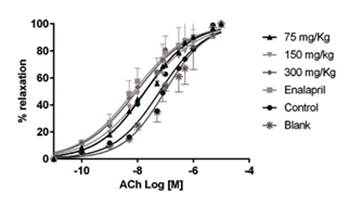
Figure 2 Cumulative (10-10 - 10-4 M) acetylcholine concentration-response curves in intact aorta rings prepared from Wistar rats previously treated with: (1) L-NAME (10 mg/kg, i.p.) plus EtOH extract of P. quadrangularis L. (75, 150, 300 mg/kg/d, p.o), (2) L-NAME plus enalapril (reference drug, 10 mg/kg/day, p.o.), (3) L-NAME plus vehicle (control: 10% propylene glycol, 10% glycerine and 2% polysorbate) and (4) vehicle witout L-NAME (blank). Results are expressed as means ± S.E.M.
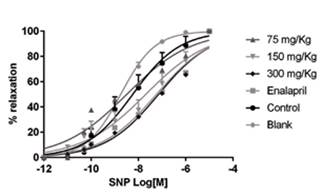
Figure 3 Cumulative (10-10 - 10-4 M) nitroprusside concentration-response curves in intact aorta rings prepared from Wistar rats previously treated with: (1) L-NAME (10 mg/kg, i.p.) plus EtOH extract of P. quadrangularis L. (75, 150, 300 mg/kg/d, p.o), (2) L-NAME plus enalapril (reference drug, 10 mg/kg/day, p.o.), (3) L-NAME plus vehicle (control; 10% propylene glycol, 10% glycerine and 2% polysorbate) and (4) vehicle without L-NAME (blank). Results are expressed as means ± S.E.M.
Table 1 pEC50 (-logEC50) induced by ACh (10-10-10-4 M) and SNP (10-10- 10-4 M) in isolated aortic rings contracted with FE (10-6 M) from Wistar rats previously treated with: (1) L-NAME (10 mg/kg, i.p.) plus EtOH extract of P. quadrangularis L. (75, 150, 300 mg/kg/d, p.o), (2) L-NAME plus enalapril (reference drug, 10 mg/kg/day, p.o.), (3) L-NAME plus vehicle (control, control; 10% propylene glycol, 10% glycerine and 2% polysorbate) and (4) vehicle without L-NAME (blank, 0.1 mL/100 g, p.o). Results are expressed as means and fiducial limits. *p<0.05 with respect to the control group.
Histopathological results
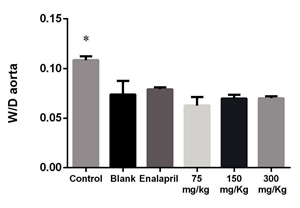
Figure 4 Estimation of aorta thickness/lumen (W/D) ratio in tissue specimens of rats previously treated with L-NAME (10 mg/kg, i.p.) plus vehicle (control), L-NAME plus P. quadrangularis L. (75, 150 and 300 mg/ kg, p.o.), L-NAME plus enalapril (10 mg/kg, p.o.) and vehicle without L-NAME (blank). *p<0.05 compared with the control.
The heart/body weight (H/BW) and wall thickness-to-diameter ratio (W/D) ratios of the control group (L-NAME) were 3.53±0.34 mg/g and 0.43± 0.02, respectively. No treatment showed any significant differences compared with this (p>0.05). However, W/D relation in aorta wall showed significant increases in the L-NAME group regarding the blank treatment (0.10±0.004 versus 0.07±0.013). This effect was reverted by P. quadrangularis L. (all doses) and enalapril (Figure 4). Masson’s trichrome staining of aorta sections from rats previously treated with L-NAME (control group) showed marked vascular fibrosis; this change was attenuated in P. quadrangularis L. (all doses) and enalapril groups (Figure 5).
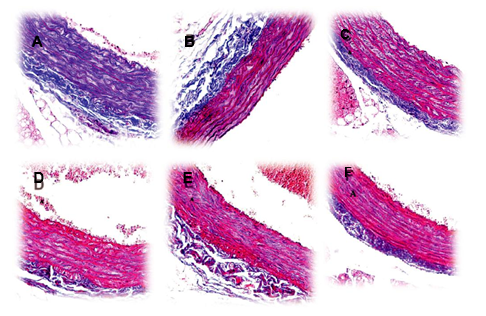
Figure 5 Representative pictures of aortic tissue sections stained with Masson’s trichrome (100µm) obtained from Wistar rats previously treated with L-NAME plus vehicle (panel A, control group), L-NAME plus enalapril (panel B, reference group), vehicle without L-NAME (panel C, blank group) and L-NAME plus P. quadrangularis L. (75, 150 and 300 mg/kg, p.o. Panels D, E and F, respectively).
DISCUSSION
This study shows that P. quadrangularis L. EtOH extract is able to prevent the hypertension induced by L-NAME in Wistar rats, increases the potency of vascular relaxant response in isolated aortic rings and attenuates aorta tissue remodelling. Until now, the antihypertensive effect of this species, administered p.o, had not been shown in an experimental model of hypertension.
The crucial role of NO in cardiovascular disorders like hypertension, coronary artery disease and heart failure has been established along with the need to improve its activity in order to attenuate the progression of related diseases 38,39. Therefore, experimental hypertension induced by L-NAME constitutes a very useful model in order to establish potential new sources for antihypertensive agents 40,41. Several antihypertensive groups, including angiotensin converting enzyme inhibitors 42, angiotensin receptor blockers 43, calcium channel blockers 44, some diuretics 45 and beta blocking drugs 46 display activity in this model. Because P. quadrangularis L. was able to prevent the hyper-tension by L-NAME (Figure 1), it has acquired interest as a potential natural source.
It is known that vascular response of isolated aorta from rats exposed chronically to L-NAME decreases 47. This work shows that P. quadrangularis L. increases the relaxant potency induced by ACh (Figure 2, Table 1), which is of endothelium-dependent nature; however, at the same time, it tends to decrease the relaxant endothelium-independent response induced by SNP (Figure 3, Table 1). These results suggest that P. quadrangularis L. possesses endothelium-dependent vasodilator metabolites, possibly related to the NO pathway, and at the same time, possible endothelium-independent vasoconstrictor compounds, but the vasodilator mechanism prevails because P. quadrangularis L. decreases blood pressure in vivo.
Remodelling is such a key phenomenon in the progression of cardiovascular disorders that agents able to ameliorate this process, including statins, ACE inhibitor, ARAII blockers, aldosterone antagonists and beta blocking drugs, can reduce the morbimortality incidence of complications like heart failure and coronary acute events, possibly at a different level 48,49,50. Hence, it is important not only to identify the antihypertensive activity of new potential sources, but to also assess its effect on remodelling models.
Among many others, L-NAME has been used in an approach to study the cardiovascular remodelling process 51. However, the dose of L-NAME needed to induce heart hypertrophy and remodelling in rats is not clear. In accordance with other works, this study did not show such changes (independent of the treatment) in histologic heart tissue samples, when 10 mg/kg of L-NAME was applied in Wistar rats 52,53. Other studies show that even higher doses and longer time intervals do not manage to elicit ventricular remodelling 54. However, at the same time, L-NAME does induce structural remodelling in aorta tissue at lower doses 36. Hence, the vascular bed seems to be more sensitive than the myocardium to NO deficit changes. Pleiotropic effects of drugs that favours NO production at endothelial level could explain, at least in part, their beneficial effects on cardiovascular remodelling, as in the case of statins 55. Interestingly, this study shows than P. quadrangularis L. is able to attenuate the aorta remodelling induced by L-NAME (Figure 4, Figure 5), adding value to its antihypertensive effect.
Angiotensin converting enzyme inhibition, a key pharmacological target for compounds for the treatment of disorders like hypertension, coronary artery disease and heart failure, has been described in P. quadrangularis L. 56. It is interesting that apigenin, a flavonoid also identified in P. quadrangularis L., elicits antihypertensive effects in spontaneously hypertensive rats in a way eventually related to up-regulating of angiotensin-converting enzyme 2 (ACE2) expression in kidney 57. Hence, apigenin could play a pivotal role in the cardiovascular properties showed by P. quadrangularis L., but other active metabolites could be implicated, as some of them have flavonoid nature, given the high phenolic content described in this species 58. Anxiolytic like properties from leaves extract of P. quadrangularis L. have also been studied in experimental models 59 and apigenin could be involved in the sedative properties showed by this species in mice, maybe be due to an enhancement of the GABAergic system 60.
Phytochemical studies of Passiflora species have led to isolation of the indole alkaloids: harmalol, harmol, harmane, harmaline and harmine, and the C-glycosyl flavonoids: orientin, isoorientin, vitexin, and isovitexin 61,62 harmol, harmane, harmaline and harmine. In addition to this kind of flavonoids, triterpene glycoside saponins, some of cycloartenol type, have also been identified in P. quadrangularis L. 63,64. Their precise role in the antihypertensive and vascular effects of P. quadrangularis L. should be assessed in subsequent works.
CONCLUSION
In conclusion, P. quadrangularis L. prevents experimental hypertension induced in rats with nitric oxide deficits improving the endothelium vasodilatation response and protecting against vascular remodelling. These results give support to the ethnobotanical use of P. quadrangularis L. as a natural antihypertensive source.














