INTRODUCTION
The emergence and rapid spread of resistant bacterial strains and the overuse of antibiotics is a significant public health problem, and the development of other strategies requires searching for new natural products that are active against pathogenic microorganisms. In recent years, several known or newly discovered bioactive molecules have been evaluated against microorganisms 1-3. In the same way, quorum sensing inhibitors are potential leads for the design of new anti-pathogenic drugs 4. A process called quorum sensing (QS) is a form of population density-dependent cell-cell communication and gene regulation that allows bacteria to coordinate settlement, virulence, luminescence, metabolite production and biofilm maturation 5,6. The interruption of QS may prevent the development of bacterial biofilms, and in this sense, it constitutes an opportunity to attenuate the pathogenicity of bacteria resistant to available antibiotics 7. In addition, promising QSI compounds have been shown to make biofilms more susceptible to antimicrobial treatments, and this indicates that a combination treatment of both QSI and antibiotics may prove useful against resistant bacteria 8.
In previous research with sponges and octocorals from the Colombian Caribbean Sea, some biologically active compounds were identified 9-11. Two cembradiene diterpenoids and other nonpolar compounds were isolated from two octocoral species of the genus Eunicea collected in Santa Marta Bay (Eunicea sp.), and they afforded only modest activity against a variety of isolated marine bacteria from immersed fouled surfaces 10,12. However, an isolated alkylglycerol (AKG), the 7 (Figure 1), showed remarkable effectiveness against biofilm formation by three of these marine bacteria, namely, Ochrobactrum pseudogringnonense, Alteromonas macleodii and Vibrio harveyi, as well as against a known biofilm-forming bacterium, Staphylococcus aureus ATCC 25923 12, but other AKGs could not be purified from these Eunicea species due to their low natural abundances.
Alkylglycerols are ether lipids naturally found in a variety of organisms such as microorganisms, fish, and invertebrates such as corals and sponges, milk, bone marrow, mammalian blood cells including human blood cells and to a lesser extent in higher plants. AKGs have multiple biological and therapeutic properties, such as the stimulation of hematopoiesis, immunological defenses, anti-tumor activity, and anti-metastasis activity as well as improving sperm quality and vaccination efficiency 13,14. Natural AKGs were found as complex mixtures of compounds that vary by length and the degree of unsaturation of the alkyl chain; however, the absolute configuration at their asymmetric carbon is always S15. Although biological testing has been done on AKGs isolated from natural sources, the separation of individual components is tedious 16, and therefore, some of the biological assays have been performed with synthesized AKGs 14. As an example, in the antibacterial activity studies, the synthetic and racemic AKG dodecylglycerol was the most potent against Enterococcus faecium, Streptococcus mutans and S. aureus17,18.
Continuing our studies on bioactive compounds against microorganisms, the aim of this work was to assess the QSI using C. violaceum of three compounds, a mixture of AKGs previously isolated from two Eunicea species, of four synthesized natural enantiomers of saturated AKGs (Figure 1) and evaluate the antibacterial activity of the AKGs against clinical isolates of bacteria, some of them known for their pathogenic capacity. The results exhibited that some compounds from those two octocorals may have potential for the control of bacterial infections.
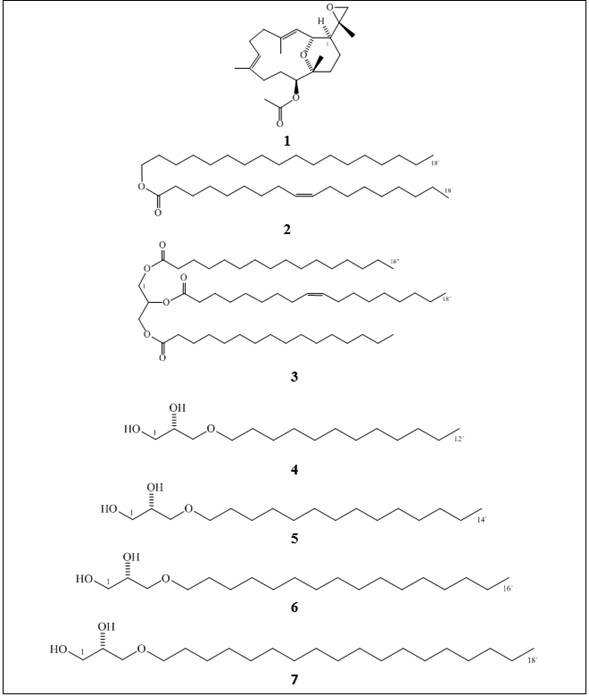
Figure 1 Chemical structures of the natural compounds and synthetic alkylglycerols (AKG) included in this study: a cembradiene diterpenoid, (+)-(1S, 2R, 11S, 12R, 15S, 3E, 7E)-11-acetoxy-2,12-oxa-15,17-epoxy-cembra-3,7-diene 1; a wax ester, stearyl oleate 2; an acylglycerol, 1,3-dihexadecanoyl-2-(9Z-octadecenoyl)-glycerol 3; the alkylglycerols (AKGs), (2S)-3-O-dodecyl-1,2-propanediol 4; (2S)-3-O-tetradecyl-1,2-propanediol 5; (2S)-3-O-hexadecyl-1,2-propanediol 6; and (2S)-3-O-octadecyl-1,2-propanediol 7.
MATERIALS AND METHODS
Reagents and equipment
The reagents (R)-(-)-2,3-O-isopropylideneglycerol ((R)-solketal), tetra-n-butyl ammonium bromide (n-Bu4NBr), sodium bis-(2-methoxyethoxy)aluminum hydride (Red-Al), kojic acid (KA) and alkyl bromides were purchased from Alfa Aesar (USA); methanesulfonyl chloride (MsCl), p-toluenesulfonic acid (p-TsOH), p-hydroxybenzaldehyde (p-HB) and methyl octadecanoate were from Merck (Germany). All reagents were of analytical grade, and solvents were dried and distilled prior to use. TLC was performed on silica gel 60 F254 (Merck) plates and visualized by UV light and 0.5 % (p/v) ceric ammonium sulfate in a 10 % sulfuric acid solution. 1H and 13C NMR spectra were recorded on an Avance 400 spectrometer (Bruker, USA) or an Avance 300, using CDCl3 and TMS as internal standards. For liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS), an LCMS-2010-ESI system (Shimadzu, Japan) was used in the positive ion mode with a RP-18 column (50 mm x 4.6 mm id x 5 µm, Waters, USA). Ten microliters of the fraction dissolved at 1mg/mL in chloroform:methanol:water:ammonium acetate 0.5:9.0:0.5:0.004 (v/v/v/v) was injected and eluted with a gradient of 9:1:0.004 methanol:water:ammonium acetate-10:0.004 methanol:ammonium acetate for 10 min at a flux of 1.5 mL/min. Additionally, 5 μL samples of pure compounds were eluted with 9:1 methanol:water-methanol. Optical rotations were measured on an ADP440 (Bellingham + Stanley, USA), and a Spectronic 20 Genesys spectrophotometer (Thermo Scientific, USA) for measuring the optical density at 600 nm was used for the evaluation of the bacterial growth.
Analysis of compounds and a fraction from two octocorals of the genus Eunicea
In our earlier works, organic compounds 1, 2, and 3 (Figure 1) and a nonpolar fraction of two octocoral species of the genus Eunicea from Santa Marta Bay, Colombia, were obtained by bio-guided isolation using an antibacterial test against several marine bacteria and they were previously assigned 10,12. In this work, the natural samples were used for QSI assays, and the nonpolar fraction was subjected to further analysis by 1H - NMR, 13C - NMR and LC-ESI-MS.
Synthesis of saturated alkylglycerols 4-7
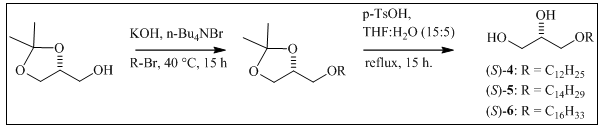
Natural saturated AKGs 4-6 were synthesized to obtain enough material for their bioassays, following the procedures described in the literature 15,16,19. To the chiral precursor (R)-solketal (0.60 g, 4.54 mmol), the corresponding alkyl bromide (1.13 g, 4.54 mmol) and n-Bu4NBr (0.29 g, 0.91 mmol), KOH (0.3 2g, 5.68 mmol) was added, and the resulting mixture was stirred for 15 hours at 40°C. The reaction was quenched by the addition of distilled water (3 mL), extracted with ethyl ether (3 x 3 mL) and washed with H2O until the pH was neutral. The organic phase was concentrated under vacuum using a rotary evaporator, and a semisolid enriched in the alkyl isopropylidene-glycerol was obtained (Scheme 1). This intermediate was deprotected in 7 mL of THF: water (15:5) with p-TsOH (55.4 mg, 0.30 mmol) and refluxed for 15 h. After extraction with chloroform (3x3 mL) and washing with H2O, the organic phase was dried with anhydrous MgSO4 and concentrated under vacuum. The obtained solid was recrystallized from hexane at 0°C to afford pure AKGs 4, 5 and 6. Each step in the synthesis was monitored by TLC.
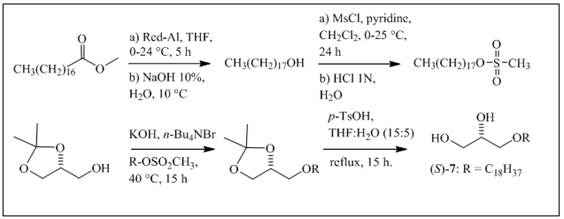
The synthesis of 7 was performed according to methods published by some authors 16,19,20. Red-Al (0.83 g, 4.15 mmol) was added to methyl octadecanoate (0.62g, 2.08mmol) dissolved in anhydrous THF (5 mL) at 0°C and stirred for 5 h at 24°C. NaOH (10% in water) was then added at 10 °C, and the organic layer was washed with water, dried and concentrated. Octadecanol was subsequently mesylated in dichloromethane with anhydrous pyridine (0.3mL, 4.53mmol) at 0°C by the addition of MsCl (0.3mL, 4.53 mmol) and stirring for 24 h at 25°C (Scheme 2). After quenching the reaction with distilled water, the organic phase was extracted with 1 N HCl, washed with water, dried, and concentrated. Finally, the newly formed mesylate was converted to AKG 7 as described above, using (R)-solketal (0.4g, 2.92 mmol), n-Bu4NBr (0.2g, 0.58 mmol), and KOH (0.3g, 5.31 mmol).
Quorum sensing inhibition assays
The QSI abilities of compounds 1, 2, and 3 as well as the fraction from Eunicea sp. were evaluated in vitro in 96-well microtiter plates (TPP, Switzerland) 6, using C. violaceum (ATCC 31532) as the biosensor 4. Pre-inoculum was cultured overnight in tryptic soy broth (TSB, Merck), and concentration was adjusted to an optical density of 0.2-0.3 at 600 nm. Then, 100 μL was added to each well, followed by 100 μL of natural samples dissolved in dimethylsulphoxide (DMSO) at final concentrations of 5, 10, 20 and 40 µg, respectively, and the plate was incubated for 24 hours at 28 °C. Thus, the QSI activity was established by the appearance of a colorless and opaque well but without affecting bacterial growth, and it was evaluated as the minimum quantity in µg per well of sample required to inhibit violacein pigment. The sample solvent (DMSO), as solvent without antimicrobial activity it served as the negative control and KA was used as the positive control. The QSI potential of synthesized AKGs was assessed using the standard disk diffusion method 21,22, with C. violaceum (ATCC 31532) and Luria-Bertani broth (LB, Merck) as growth medium. Sterile filter paper discs 5 mm in diameter (Whatman, USA) were impregnated with 10, 20, 30 and 50 μg of each AKG dissolved in dichloromethane (DCM) and dried at room temperature for 20 minutes. The inoculum was adjusted to 106 CFU/mL (0.5 McFarland) in LB agar in Petri dishes. The discs were placed on the agar, the plates were incubated for 24-48 h at 27°C and the inhibition halo was measured. This assay also measures the minimum amount of each compound that inhibits pigment production (violacein) around the disc and indicates that the QS of C. violaceum was disrupted but does not interfere with bacterial growth 22. As positive controls, KA 5, and p-HB 28, were used at the same concentrations and treated as described above per disc, and DCM as inactive solvent to antimicrobial activity was used as a negative control.
Evaluation of the antibacterial activity
To further investigate the antibacterial properties, the synthesized AKGs were selected since all natural compounds were isolated from these octocorals in low quantities. Their MICs were determined in vitro in microtiter plates against 14 clinical bacterial isolates 23-25. Each AKG was dissolved in dimethylformamide (DMF) (1 mg/mL) to final concentrations of 1, 2, 4, 8, 16, 32, 64, 128, 256 and 512 μg/mL in wells. Bacterial strains were grown with Mueller-Hinton agar (MHA, Merck). The inoculum (100 μL, 104 CFU/mL) in each well was mixed with the AKG in MH broth to 200 μL, and the plates were incubated at 37°C for 18-24 h. An inoculated well with DMF in culture medium and blank well containing only assay medium were used as growth controls. The MIC of 64 μg/mL of racemic dodecylglycerol against S. aureus18) was used as a standard reference data point. Among the clinical isolates, the gram-positive bacteria Listeria innocua, Staphylococcus aureus, Enterococcus faecalis, Brevibacillus brevis, and Micrococcus luteus as well as the gram-negative bacteria Escherichia coli, Salmonella enteritidis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Serratia marcescens, Enterobacter agglomerans, Klebsiella oxytoca, Acinetobacter baumannii, and Proteus mirabilis were provided by the Hospital of Neiva (Huila), Hospital of Tunal, Hospital of Engativá and Universidad del Bosque (Bogotá).
Statistical analysis
Bioassay determinations were replicated three times and reported as the mean values using the standard error and analysis of variance ANOVA’s Tukey test. A difference was considered statistically significant when p<0.05 26.
RESULTS
Analysis of compounds and a fraction from two octocorals of the genus Eunicea.
In this work, natural compounds and a nonpolar fraction were reserved for QSI assays. The nonpolar fraction was found by NMR to be a mixture of AKGs, and by LC-MS-ESI, it was found to have the abundant ions [M + Na]+, [M + H]+, and [M + NH4]+ (Figure 2).
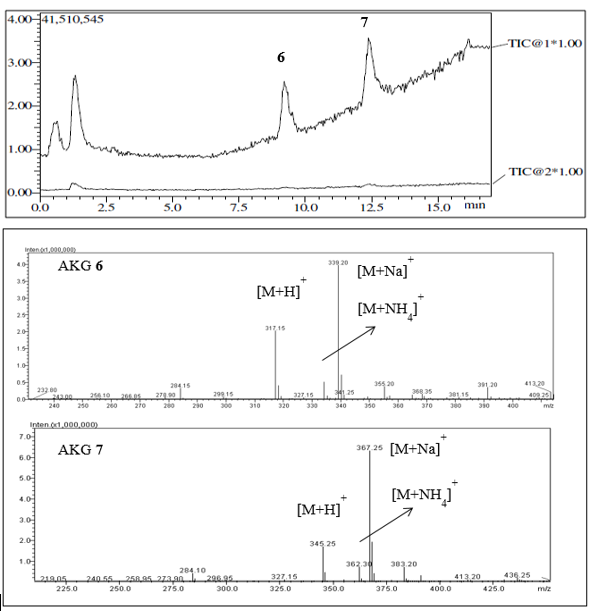
Figure 2 TIC (total ion chromatogram) obtained by LC-MS-ESI of a fraction from Eunicea sp., showing the separation and spectra of the major identified AKGs 6 and 7.
Synthesis of saturated alkylglycerols 4-7
Chemical structures of the synthesized AKGs were confirmed by NMR and LC-ESI-MS spectra along with the specific optical rotations as follows.
Compound 4. Yield 50% as a bright white solid, (560 mg, 2.15 mmol). 1H NMR (400 MHz, CDCl3/TMS): δ 3.88-3.82 (m, 1H, H-2), 3.70 (dd, J = 11.4, 3.8 Hz, 1H, H-1a), 3.63 (dd, J = 11.4, 5.4 Hz, 1H, H-1b), 3.52 (dd, J = 9.8, 4.0 Hz, 1H, H-3a), 3.48 (dd, J = 9.8, 5.9 Hz, 1H, H-3b), 3.45 (2x dt, J = 9.1, 6.7 Hz, 2H, H-1´), 2.56 (s, 2H, CH2OH x 2), 1.56 (br. quint, J = 6.8 Hz, 2H, H-2´), 1.35-1.16 (m, 18H, CH 2 x 9), 0.87 (t, J = 6.8 Hz, 3H, H-12´) ppm. 13C NMR (100 MHz, CDCl3): δ 72.60 (C-3), 72.00 (C-1´), 70.64 (C-2), 64.40 (C-1), 32.05 (C-10´), 29.79, 29.76, 29.74, 29.72, 29.59, 29.48 (7C, C-2´ and C-4´-C-9´), 26.21 (C-3´), 22.82 (C-11´) 14.24 (C-12´). LC-MS-ESI m/z 261.05 [M + H]+ (34%), 283.05 [M+ Na]+ (100%). [α]20 D +2.2 (c 4.3, CHCl3).
Compound 5. Yield 52% as a white solid (385 mg, 1.33 mmol). 1H NMR (400 MHz, CDCl3/TMS): δ 3.89-3.83 (m, 1H, H-2), 3.71 (dd, J = 11.4, 3.8 Hz, 1H, H-1a), 3.63 (dd, J = 11.4, 5.3 Hz, 1H, H-1b), 3.52 (dd, J = 9.7, 4.0 Hz, 1H, H-3a), 3.48 (dd, J = 9.7, 6.1 Hz, 1H, H-3b), 3.45 (2x dt, J = 9.3, 6.7 Hz, 2H, H-1´), 2.80 (br. s, 1H, CHOH), 2.40 (br. s, 1H, CH2OH), 1.57 (br. quint, J = 6.8, 2H, H-2´), 1.25 (br. s, 22H, CH 2 x 11), 0.87 (t, J = 6.8 Hz, 3H, H-14’). 13C NMR (100 MHz, CDCl3): ( 72.62 (C-3), 72.00 (C-1´), 70.60 (C-2), 64.40 (C-1), 32.06 (C-12´), 29.83, 29.81, 29.80, 29.75, 29.72, 29.71, 29.59, 29.50 (9C, C-2´ and C-4´-C-11´), 26.21 (C-3´), 22.83 (C-13´), 14.26 (C-14´). LC-MS-ESI m/z 289.10 [M + H]+ (40%), 311.10 [M + Na]+ (100%). [α]20 D +2.1 (c 4.1, CHCl3).
Compound 6. Yield 27% as a white greasy solid (307 mg, 0.97 mmol). 1H NMR (300 MHz, CDCl3/TMS): δ 3.89-3.81 (m, 1H, H-2), 3.70 (dd, J = 11.4, 3.8 Hz, 1H, H-1a), 3.64 (dd, J = 11.4, 5.4 Hz, 1H, H-1b), 3.55-3.49 (m, 1H, H-3a), 3.51-3.46 (m, 1H, H-3b), 3.47-3.40 (m, 2H, H-1´), 2.20 (br. s, 2H, CHOH x 2), 1.59-1.52 (m, 2H, H-2´), 1.25 (br. s, 26H, CH 2 x 13), 0.87 (t, J = 6.7 Hz, 3H, H-16´).13C NMR and 13C NMR APT (75 MHz, CDCl3) δ 72.57 (C-3), 71.98 (C-1´), 70.66 (C-2), 64.38 (C-1), 32.05 (C-14´), 29.82, 29.79, 29.74, 29.72, 29.60, 29.49 (11C, C-2´and C-4´-C13´), 26.21 (C-3´), 22.81 (C-15´), 14.23 (C-16´). LC-MS-ESI m/z 317.20 [M + H]+ (20%), 339.15 [M + Na]+ (100%). [α]20 D +2.5 (c 3.7, CHCl3).
Compound 7. Yield 25% as an amorphous solid (180 mg, 0.52 mmol). 1H NMR (400 MHz, CDCl3/TMS) δ 3.90-3.83 (m, 1H, H-2), 3.71 (dd, J = 11.5, 3.7 Hz, 1H, H-1a), 3.64 (dd, J = 11.5, 5.4 Hz, 1H, H-1b), 3.53 (dd, J = 9.9, 4.3 Hz, 1H, H-3a), 3.49 (dd, J = 9.9, 6.4 Hz, 1H, H-3b), 3.46 (2x dt, J = 9.5, 6.8 Hz, 2H, H-1´), 2.85 (br. s, 1H, CHOH), 2.05 (br. s, 1H, CHOH), 1.57 (br. quint, J = 6.8 Hz, 2H, H-2´), 1.25 (br. s, 30H, CH 2 x 15), 0.88 (t, J = 6.8 Hz, 3H, H-18´). 13C NMR and 13C NMR APT (100 MHz, CDCl3), δ 72.58 (C-3), 72.00 (C-1´), 70.66 (C-2), 64.37 (C-1), 32.07 (C-16´), 29.85, 29.82, 29.77, 29.75, 29.74, 29.71,29.62, 29.51 (13C, C-2´and C-4´-C-15´), 26.22 (C-3´), 22.84 (C-17´), 14.27 (C-18´). LC-MS-ESI m/z 345.25 [M + H]+ (31%), 367.25 [M + Na]+ (100%). [α]20 D +2.7 (c 4.0, CHCl3).
Quorum sensing inhibition assays
Cembradiene 1 and the mixture of AKGs presented inhibition at the same concentration as KA, the control, while 2 and 3 were less active (Table 1). It was observed that neither AKGs affected the growth of C. violaceum.
Table 1 Quorum sensing inhibition of an AKG mixture and compounds 1, 2, and 3 isolated from Eunicea sp. in C. violaceum (ATCC 31532) by the microtiter plate assay.

aMinimum quantity in µg of compound per well required to inhibit violacein pigment. bKA was used as a positive control. cDimethylsulphoxide (DMSO) was used as a negative control. --, No inhibition was observed in the assay conditions. Data represent the mean from three independent experiments. *The ANOVA showed a statistically significant difference between the controls and treated groups (p < 0.05).
Moreover, the results of QSI showed that AKG 4 exhibited the same minimum inhibition halo of 14 ± 1 mm at 20 μg/disc as KA, while AKG 5 and the other control, p-HB gave a halo of 12 ± 3 mm at 50 μg/disc (Figure 3, Table 2). The C. violaceum growth confirmed that only they inhibited violacein production.
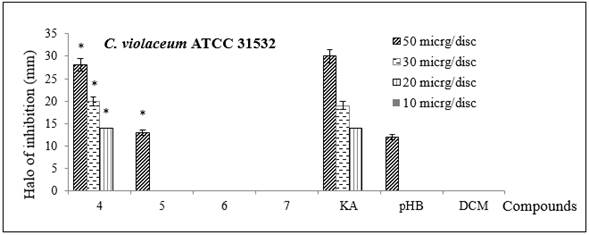
Figure 3 Inhibition halo (in mm) of QSI by the disc diffusion assay of synthesized alkylglycerols 4-7 in C violaceum (ATCC 31532). KA and p-HB were positive controls. DCM was a negative control. Bars indicate the standard deviation of three replicates. Statistically significant differences (p < 0.05, ANOVA) between the treatment and the negative control (without AKG) are marked by asterisks.
Table 2 Quorum sensing inhibitory activity of synthesized alkylglycerols 4-7 against the biosensor strain C. violaceum (ATCC 31532) by the disc diffusion assay.
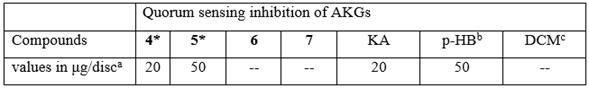
aMinimum quantity in µg of compound per disc required to inhibit violacein pigment. bKA and p-HB were used as a positive control. cDichloromethane (DCM) was used as a negative control. --, No inhibition zone observed in the assay conditions. Only the synthesized AKGs 4-7 were selected for this assay since all natural 1, 2, 3 compounds were isolated from these two octocorals in insufficient amount. Data are the mean of three separate experiments. *The ANOVA showed a statistically significant difference relative to controls (p < 0.05).
Evaluation of the antibacterial activity
The results show that AKGs 4 and 5 were the most active against five of the gram-positive bacterial strains (Table 3), while against the gram-negative strains S. enteritidis, K. pneumonia, E. agglomerans, K. oxytoca, and A. baumannii, the AKGs were inactive. However, AKG 4 was active against P. aeruginosa and P. mirabilis at the highest concentration assayed.
Table 3 Growth inhibitory activities of synthesized alkylglycerols (AKGs) 4-7 against clinical bacterial isolates by the microtiter plate assay.
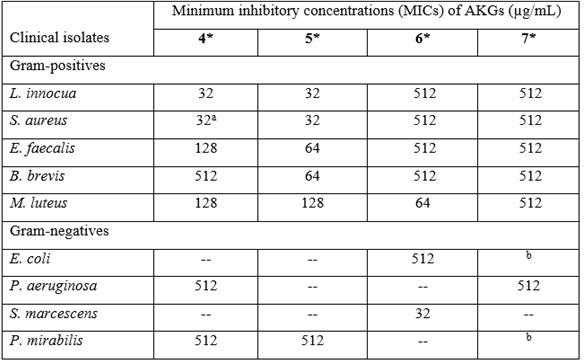
aRacemic dodecylglycerol has an MIC of 64 μg/mL against S. aureus17, and this was used as a reference value. A treatment with DMF in culture medium without compound was used as bacterial growth control, while the treatment containing only assay medium served as blank control. --, Not detected under the assay conditions. Data represent the mean of three different experiments. *The ANOVA revealed significant differences (p < 0.05) between the tests and the growth control of each of these bacteria. bNo significant difference was observed between treated and the bacterial growth control.
DISCUSSION
In this work, natural compounds 1, 2, and 3 as well as the AKG mixture were subjected to QSI assays. The LC-MS-ESI of the AKG mixture had ions corresponding with molecular masses of 316 g/mol and 344 g/mol, respectively, and thus, 6 and 7 were identified in a 1:2 ratio as the major AKGs, along with other probable saturated AKGs with lower masses (Figure 2). Recently, only 7 had been purified 12, and unfortunately, we were unable to investigate the effects of all compounds from the two octocorals in the QSI and antibacterial assays because some of them were available in low quantities. Particularly, alkylglycerols (AKGs) are found in low concentrations in marine sponges and corals and at trace levels in mammal, including humans 16. Previous studies were developed with the most abundant AKGs or their mixtures, and recent advances in AKG synthesis have been crucial for the exploration of their biological activities 13,14. In this work, the most common natural enantiomers of saturated AKGs 4-713, were synthesized (Figure 1). One of the most suitable and well-established routes for their preparation is from a chiral precursor with an alkyl halide or sulfonate 15,16,19,20. Thus, yields of the AKGs were satisfactory, ranging from 25 % to 52 %, as no further chromatographic purifications were performed, and sufficient material was obtained for the biological tests. Their chemical structures were confirmed by spectroscopic data with those previously published. Mass spectrometry analysis of compound 4 by LC-MS-ESI showed the followed two peaks: m/z 261.05 (34 %) corresponding to the ion [M + H]+ and m/z 283.05 (100 %) corresponding to the ion [M + Na]+, which are consistent with a molecular mass of 260.05 g/mol and formula of C15H32O3. The positive optical rotation, [α]20D +2.2 (c 4.3, CHCl3), is typical of the S configuration of the natural AKGs 16, confirming its optically purity, and the NMR data were similar to those obtained for AKGs 6 and 7, allowing 4 to be assigned as (2S)-3-O-dodecyl-1,2-propanediol, a natural AKG previously synthesized 13. Compound 5 had the formula C17H36O3, by LC-MS-ESI. Its optical rotation and NMR spectra were consistent with those of 4, and it was confirmed to be (2S)-3-O-tetradecyl-1,2-propanediol, a natural and previously synthesized AKG 13. Compound 6 was C19H40O3, its optical rotation and NMR data are similar to those of the AKG synthesized 15, and thus, it was named (2S)-3-O-hexadecyl-1,2-propanediol, a natural compound known as chimyl alcohol. Compound 7 had the formula C21H44O3, and its optical rotation and NMR data are in agreement with those of the natural batyl alcohol 12 previously synthesized 15; therefore, it was assigned as (2S)-3-O-octadecyl-1,2-propanediol.
In relation to the natural samples tested for QSI by the microtiter plate assay, compounds 1, 2, and 3 as well as the mixture of AKGs display activity. 1 and the AKG mixture presented the largest inhibition at the same concentration as the positive control, KA (10 µg/well), while 2 and 3 were less active. Compound 1 was first isolated from a specimen of Eunicea collected near Providencia Island and displayed antiplasmodial activity against Plasmodium falciparum27. In our recent research, we isolated 1 again from Eunicea sp. collected in Santa Marta Bay, and its absolute configuration was determined, but it showed weak antimicrobial activity against marine bacteria 10. In this work, 1 displayed significant QSI and was as active as KA, the positive control used and a known inhibitor of QS systems 5. Other Eunicea and Pseudoplexaura cembranoid diterpenes have shown prominent QSI activity 11. Surprisingly, the nonpolar fraction was as active as KA or as 1, and since this fraction is a mixture of alkylglycerols, it indicated that natural AKGs could be interesting QS inhibitors, which was another reason that motivated us to synthesize some of its most likely constituents, four of the major natural saturated alquilgliceroles 13. Compound 2 was previously biosynthesized and it has been detected in the eyelids of humans and mammalians. It was isolated from Eunicea sp. and showed biofilm inhibition up to 91 % against S. aureus ATCC 25923 12. 3 only has been isolated from Eunicea sp., and it was effective against biofilm formation by V. harveyi (Phy-2A) and P. aeruginosa (ATCC 27853), with a percentage higher than 25 % 12. In this work, compounds 2 and 3 showed QSI at 20 and 40 µg/well, respectively, but they were less active than KA.
When synthetic AKGs were evaluated by the disc diffusion assay, 4 was the most active, exhibiting the same QSI as KA (20 µg/disc), followed by 5, which had the same QSI as p-HB at 50 µg/disc, all of them without affecting the microbial growth of C. violaceum (ATCC 31532). Meanwhile, 6 and 7 were practically inactive to the concentrations assayed. AKGs 4 and 5 could be responsible for the QSI activity determined in the AKG mixture from Eunicea sp. In addition to KA, p-HB was selected as a control because it demonstrated QSI against C. violaceum (ATCC 31532) 28, and it is a natural component of vanilla extract, which is also a QS inhibitor 29. Other nonpolar and natural compounds that can interfere with QS include, furanones, chlorinated metabolites (called honaucins), unsaturated lactones (nocapyrones), furanosesquiterpenoid (felixinin), sesquiterpene alcohol (farnesol), unsaturated fatty acids (pitinoic acid and cis-9-octadecenoic acid), and a cyclopropyl fatty acid (lyngbyoic acid) 30,31. In the current work, the bioactive natural and known compounds, 1, 2, 3, 4 and 5 displayed in vitro QSI for the first time.
The antibacterial potential of the synthesized AKGs was evaluated against clinical isolates of bacteria, and the MICs were determined in a microtiter plate assay. In this way, 4 and 5 showed in vitro antibacterial activity against five of the gram-positive strains: L. innocua and S. aureus (MICs of 32 μg/mL), with less effectiveness against E. faecalis, B. brevis and M. luteus. The AKGs, 4 and 5, were much less active against gram-negative clinical isolates, as shown in Table 3. These AKGs showed bacteriostatic effects on the clinical isolates because evaluation of the viability by seeding in fresh culture medium led to the recovery of each bacterial species (data not shown). The results from this study are consistent with previous observations for synthesized and racemic AKGs with chains of 8, 10, 12, 14 and 16 carbons, and among them, racemic dodecylglycerol was an effective antibacterial against the gram-positive strains of S. aureus, E. faecium, and S. mutans. It also showed low activity against the gram-negative bacteria P. aeruginosa and K. pneumonia17,18. However, in the present work, a MIC value of 32 μg/mL was found for the pure enantiomer 4 against S. aureus, which was better than that of racemic dodecylglycerol with the published value of 64 μg/mL 17. Nevertheless, previously 7 showed low activity against the gram-negative marine bacteria Pseudoalteromonas piscida, Ruegeria sp., Vibrio alginolyticus, V. furnissii and V. harveyi32. We have reported that 7 isolated from Eunicea sp. had moderate activity against the gram-negative marine bacteria O. pseudogringnonense (4-4DEP) and A. macleodii (29-C), the reference strain P. aeruginosa ATCC 27853, and even the gram-positive S. aureus ATCC 25923 12. Many other natural lipids show antimicrobial activity 14,17,30, but the considerable resistance of gram-negative clinical isolates to AKGs could be attributed to their outer membrane, which creates a protective barrier against hydrophobic compounds such as lipids 33. Among the strains that were susceptible to AKGs, L. innocua, which is widely distributed in nature, is able to overcome extreme conditions of pH, temperature, and salinity, and although it is not considered pathogenic, it has caused some infections in humans requiring treatments with prolonged doses of antibiotics 34. Its powerful ability to grow in certain foods has been suggested to be an expression of QS 35. S. aureus is a widespread pathogen and known food contaminant that has developed antibiotic resistance and can cause a variety of QS-regulated infectious diseases 36,37. E. faecalis is a multiantibiotic resistant pathogen that resides in the human gastrointestinal tract, but studies have proven the potential for developing QS antagonists that could help control this pathogen 38. M. luteus has been identified from several sources, such as human tissues, and although it is not considered pathogenic, is it characterized by withstanding various environmental conditions 39. B. brevis is found in soils, air, and water, and it exhibits moderate resistance 40.
Present research provides additional information. It constitutes the first report of the QSI activity of natural compounds 1, 2, 3, 4 and 5. Additionally, the optically pure AKGs 4 and 5 showed more potent antimicrobial activity against L. innocua and S. aureus with a MIC of 32 μg/mL, and they were active against E. faecalis, B. brevis, and M. luteus.
The main limitation of this research was that the natural compounds were available in low quantities; however, these compounds may be an alternative for new studies against pathogenic microorganisms, and the results motivated us to produce other natural AKGs because of their relatively simple structures and short chemical synthesis. Also, these advances open the possibilities to obtain by synthesis that class of compounds, specifically the alkylglycerols, since they have already found some applications in several industrial and pharmaceutical domains and could be considered as promising compounds either from an economic or an ecological point of view 13,14.
CONCLUSIONS
This study illustrated, for the first time, the importance of bioactive natural compounds 1, 2, 3, and AKGs 4 and 5 as QS inhibitors in C. violaceum (ATCC 31532). The naturally occurring enantiomers of four AKGs (4-7) were synthesized. Cembradiene 1 together with the AKG mixture from Eunicea and AKG 4 inhibit QS at the same concentration as KA. In addition, 4 and 5 displayed specific antibacterial activity against gram-positive clinical isolates in the following order: L. innocua and S. aureus (MIC = 32 μg/mL for both 4 and 5), E. faecalis (128 µg/mL and 64 µg/mL respectively), M. luteus (128 µg/mL for both) and B. brevis (512 µg/mL and 64 µg/mL respectively). The interesting results show that additional studies are necessary to explore the prospects of controlling pathogenic microorganisms with these natural compounds or other natural AKGs obtained by synthesis.