INTRODUCTION
Major depression is a potential serious disorder with a high impact in worldwide public health, affecting up 20% of the population, and contributes to consequential disabling in personal development, social relations, and higher morbidity and mortality (1). In Colombia, mental disorders reach up to 40% and major depression is the most prevalent (2). Given that this disorder results from the conjunction of a variable proportion of factors of endogenous nature, added to exogenous triggers, the appropriate psychological approach combined with pharmacological treatment are the backbone of therapy to achieve a greater improvement in patient response (3, 4).
Pharmacological treatment for major depression includes: selective serotonin reuptake inhibitors (SSRIs), serotonin/norepinephrine reuptake inhibitors (SNRIs), atypical antidepressants, serotonin-dopamine activity modulators (SDAMs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and St. John’s wort. Although evidence for St. John’s wort’s effectiveness is modest, overall, effectiveness of the different antidepressant groups appears be similar, albeit, there are differences with respect to onset of action and adverse events profile (5).
Reducing neurotransmitter breakdown through pharmacological inhibition of central monoamino oxidase (MAO), has been an effective strategy for treatment of a broad range of affective and anxiety disorders. However, the risk of hypertensive crisis discouraged the use of non-selective MAO inhibitors and led to the search for selective and non-reversible agents, such as selegiline, a MAO-B inhibitor, usually prescribed for Parkinson disease, but also effective for major depression in a transdermal system (6).
MAO-A selective inhibition has been another approach to get an antidepressant effect with lower risks of potentiation of cardiovascular effects of dietary amines (7). Moclobemide is the prototype agent of this group that attained clinical use in several countries, but newer MAO-A inhibitors could represent an alternative treatment (8).
Coumarin is an active metabolite, isolated from many medicinal plants, among them, Hygrophila tyttha (“amansamachos”), used popularly in Colombia as a tranquilizer (9, 10, 11). This natural metabolite has served as the base for obtaining derivatives directed to different pharmacological targets, among which, MAO inhibition is one of the main targets (12). Several coumarin derivatives with a variety of ring substitutions, including 3-phenyl (13, 14), 3-aryl (15, 16), and 7-benzylpiperidin coumarins (17, 18) have led to potent MAO-B inhibition, whereas 6-amino 3-pyrrolidin (19) and 7-oxy-coumarins (20) have led to MAO-A inhibition. However, the correlation of the biological activity between coumarin derivatives in vitro and in vivo is a matter that requires further optimization (21).
Previous work showed that the coumarin derivative, coded as FCS-304 (4-propyl-2Hbenzo[h]-chromen-2-one) displays MAO-A inhibition at the micromolar range, with no activity against MAO-B, as well as positive results in forced swimming and tail suspension test in mice (22). This work advances the antidepressant-like profile of this compound, assessing its activity in the 5-HTP test in mice to explore its pro-serotoninergic activity, as well as its response in rats; in reserpine induce ptosis and in the behavioral despair test.
MATERIALS AND METHODS
FCS-304 synthesis
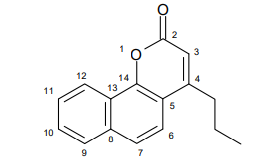
FCS-304 was obtained according to previously described, following Pechman condensation 22,23, starting from 1-naphthol and ethyl 3-oxohexanoate (yield of 80%). FCS-304 melting point values were 103 - 105 ºC and infrared and 1H, 13C magnetic resonance spectra leading to the 4-propyl-7,8-benzocoumarine structure (Figure 1, C16H14O2, M.W: 238).
Experimental protocol
Animals were supplied by the Bioterium of the pharmacy department, Faculty of Sciences, Universidad Nacional de Colombia. Male ICR mice and female Wistar rats of 9-11 weeks old were kept under a 12 h dark/light cycle, 22ºC temperature and 70% moisture with free access to water and food, except during the experiment day. They were randomly assigned to the following treatment groups (n=6-7 per group): FCS-304, three doses: 50, 75, and 150 mg/kg, p.o., according to a previous study (22), imipramine, as reference agent, 30 mg/kg, p.o. and vehicle: canola oil, as control group (0.1 mL/10 g, p.o.). These treatments were administered 1 h before each test. The experimental procedure was approved by the institutional ethics committee (Act 4, Jun 9, 2014, Faculty of Sciences, at the Universidad Nacional de Colombia).
5-HTP-induced head twitches in mice
This test was performed, based on Koe et al., (24, 25), administering 5-HTP, 100 mg/kg, i.p. in mice treated 1h prior with FCS-304, imipramine, or vehicle, according to the experimental protocol described above. Number of head twitches per animal was registered during 60 min.
Behavioral despair test in rats
This test was performed, based on Porsolt et al, (26, 27). Rats previously treated with FCS304, imipramine, or vehicle were placed in a tank containing water (55 cm deep, 25°C) and left there for 5 min, measuring the total duration of immobility when the animals made only movements necessary to keep their heads above water.
Reserpine inducedptosis in rats
This test was performed based on Worms et al, (25, 28). Rats previously treated with FCS-304, imipramine or vehicle were injected with reserpine, 3.6 mg/kg, i.p. (according to preliminary assays) and 2 h later, the degree of ptosis was evaluated as follows: 0, eyes open; 1, eyes one-quarter closed; 2, eyes half closed; 3, eyes three-quarters closed; 4, eyes completely closed.
Drugs and solutions
Imipramine, L-5-hydroxytryptophan (5-HTP) and reserpine were obtained from Sigma®. Imipramine was dissolved in SSN, 0.9% (3 mg/mL), FCS-304 (4-propil-2H-benzo[h]- cromen-2-ona) was suspended in a canola oil vehicle and reserpine was prepared in a vehicle formed by a mixture of polysorbate-80, polypropyleneglycol-400, glycerin, and distilled water, in proportions of 2, 10, 10, and 78.
Statistical and data analysis
Results are expressed as mean ± standard error mean (S.E.M.). Given the small number of samples, Kolmogorov Smirnov test was applied to assess Gaussian distribution. Then, one-way ANOVA or Kruskall Wallis analysis, followed by multiple comparisons test were applied (p≤0.05 against control). GraphPad-Prism ® software, version 6, was used for data analysis.
RESULTS
Effects of FCS-304 in 5-HTP-induced twitches in mice
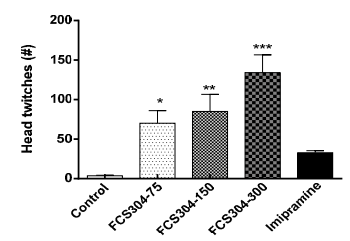
FCS-304, administered as a dose of 75, 150, and 300 mg/kg, p.o., significantly increased the number of mice head twitches in a dose dependent manner. Imipramine showed a slight increase (Figure 2).
Effects of FCS-304 in behavioral despair in rats
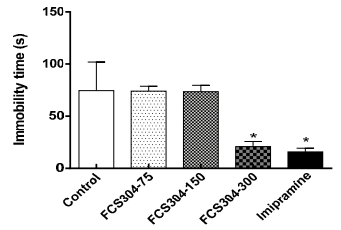
Behavioral despair in rats, under induced forced swimming, showed a significant decrease in immobility time in rats treated with FCS-304, 300 mg/kg and the reference agent, imipramine, 30 mg/kg. Medium and low dose of FCS-304 (75, 50 mg/kg) did not achieve any difference from the control (Figure 3).
Effects of FCS-304 in reserpinized rats
FCS-304 significantly decreased the palpebral ptosis and akinesia induced by reserpine in a dose dependent manner. Imipramine showed a greater response (Figure 4).
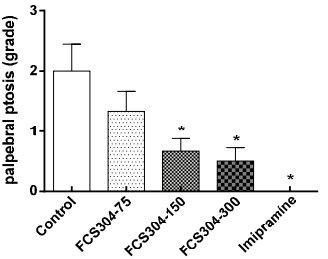
Figure 4 Effect of FCS-304 (75, 150, and 300 mg/kg., v.o.), imipramine (30 mg/kg, v.o.) and control (vehicle, 0.1 mL/10 g., v.o.) in the grade of palpebral ptosis induced by reserpine (3.6 mg/kg, i.p.) in rats. Values are represented as: 0, eyes open; 1, eyes one-quarter closed; 2, eyes half closed; 3, eyes three-quarters closed; 4, eyes completely closed *p<0.05, against control.
DISCUSSION
This study shows that FCS-304 increased the pro-serotoninergic mechanism linked to the 5-OH-triptamine precursor 5-HTP, manifested by exacerbation of head twitches in mice, whereas in rats, it reduced the immobility time in forced swimming and decreased palpebral ptosis induced by reserpine. Globally considered, these results support the idea that FCS-304 follows an antidepressant like profile.
A previous work showed that FCS-304 displays an IC50 value of micromolar range against hMAO-whereas it lacks activity against hMAO-B (22). This selective inhibition of MAO-A isozyme by FCS-304 would represent the advantage of a less adverse effect profile related to cardiovascular risk, given that tyramine, present in foods like cheese and wine, shows similar affinity for each enzyme form, and hence, could be metabolized by MAO-B (7, 29).
Previously FCS-304 had showed activity in forced swimming and tail suspension test in mice, therefore, it was useful to explore the activity of FCS-304 in other experimental models of depression and in another species, in this case, the rat. 5-HTP, as the immediate precursor in the biosynthesis of serotonin from tryptophan, is useful to study pro-serotoninergic mechanisms of potential drugs and it is especially useful to test MAO-A inhibitors (30, 31).
Results of the 5HTP test support that FCS-304 could act through a pro-serotoninergic mechanism at the central level. In addition, given that forced swimming and reserpine tests are high sensitive to non-selective agents, among them, tricyclic antidepressants (32, 33), it is reasonable to assume that imipramine shows a greater response in both assays, whereas FCS-304 is more effective against 5-HTP.
FCS-304 would share the favorable profile of selective MAO-A inhibitors: an anti-depressive profile linked to an increase in the activity of central serotonin, noradrenalin, and dopamine, neurotransmitters that participate in the regulation of the mood, whereas at the same time, have less risk of hypertensive events related to foods containing tyramine (7). Furthermore, MAO-A inhibition, due to its capacity to lower the generation of free radicals and toxins, remains as a target in the search for new drugs for the treatment of neurologic and neurodegenerative disorders (34, 35). This fact increases the pharmacological interest of FCS-304.
Coumarin derivatives have been extensively studied as sources of compounds with potential applicability in a wide range of disorders, including those of neurological nature: depression, anxiety, chronic pain, and Alzheimer disease, among them. Potent MAO-B and MAO-A coumarin inhibitors have been developed, but correlation between in silico, in vitro, and in vivo requires further optimization (12, 21).
Regarding FCS-304 as a MAO-A selectivity inhibitor, it was suggested that 4-hydrogen substitution and 7 aromatic radical presence, which could lead to covalent interactions with the flavin ring of the FAD cofactor required by the isozyme, would contribute the mechanism of action of this compound, but more research is needed in order to determine its structure-activity relationship (22).
CONCLUSION
In conclusion, these results support the proposal that FCS-304 is a coumarin derivative with MAO-A selective properties and antidepressant-like effects in mice and rats. More studies, including those of safety profile, are required to advance the pharmacological interest of this compound.