INTRODUCTION
Toxinology is a sub-field of Toxicology, and it is in charge of the study of substances produced by living organisms, either delivered as venoms, or residing within the tissues of animals, plants, fungi, and bacteria, and which may harm target organisms (1, 2). Thus, Toxins are substances produced by living organisms, and they can impede the normal functioning of organisms that are exposed to them. Venoms are mixtures of proteins, enzymes, or other molecules produced in glands and secreted through specialized systems (spines, fangs, etc.) in order to immobilize prey or to provide defense against predators. In contrast, poisons are substances that are concentrated in the bodies of some organisms, or certain parts of them, and they can cause deleterious effects if they are handled or eaten (1). Consequently, both venoms and poisons are composed of toxins, Toxinology studies both.
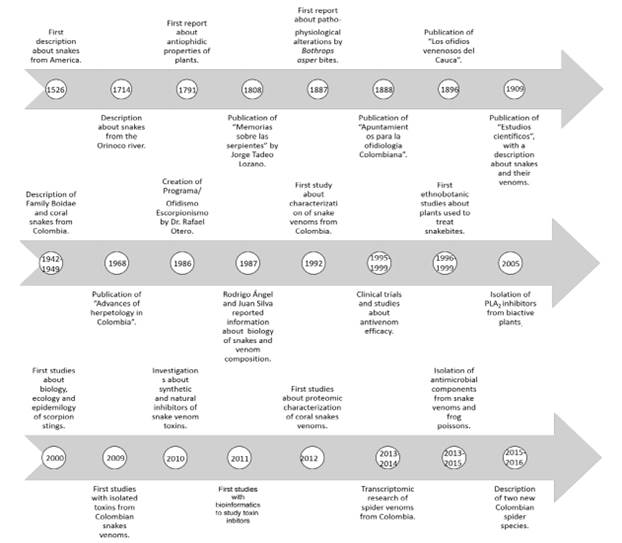
On the other hand, Clinical Toxinology is a sub-field of Toxinology, and it studies the harmful effects of toxins, either from venomous or poisonous animals, plants, or fungi. Finally, Antivenoms are antibodies produced to neutralize toxins present in venoms or poisons. Hiperimmunizing some animals produce the antivenoms -mainly horses-, although other animals such as sheep or camels can be employed for this purpose. Antivenoms can be monovalent when only one venom is used to immunize the animal, or polyvalent, when several venoms are used to immunize the animal (3). In this work, a brief history of ophidiology and toxinology advances in Colombia is presented. The timeline of this study is shown in Figure 1.
A brief history of ophidiology in Colombia
The first descriptions of snakes in our continent date from 1526, when Gonzalo Fernández de Oviedo sent by the Spanish authorities to America and could publish a manuscript entitled “General and natural history of the Indies, islands, and mainland” (4). However, it was not until 1714, when Joseph Gumilla published his work entitled “El Orinoco Ilustrado: historia natural, civil y geográfica de este gran río y de sus caudales vertientes”, with more specific information about snakes that inhabited the Orinoco River versant. For example, Gumilla reported the presence of venomous snakes, such as rattlesnakes, in the eastern plains of Colombia. Besides, he described some traditional practices followed to treat snakebite (5). In 1791, Pedro Fermín de Vargas informed in the Santa Fé de Bogotá Newspaper about the antiophidic properties of guaco plant (Mikania sp). Such properties were initially discovered by people of the western rainforest region of Colombia (Chocó department) while observing that a bird also called guaco or laughing falcon (Herpetotheres cachinnans) used to ingest the juice of the guaco plant, and then hunt snakes and endure their bite (6). In 2000, Otero et al. (7) informed about the traditional use of Mikania guaco in the northwestern region of Colombia, confirming that knowledge about the antiophidic properties of the plant had been transmitted from generation to generation. In 1808, Jorge Tadeo Lozano published his work entitled “Memorias sobre las Serpientes”, which included specific information about venomous and non-venomous snakes, empiric treatments for snakebite, and, once again, the use of guaco (8). In 1887, José Restrepo described, for the first time, pathophysiological alterations in a patient bit by a Bothrops asper (9). Similarly, in 1889, Posada-Arango published a work entitled “Apuntamientos para la ofidiología Colombiana”, in which he showed a detailed description of snakes from the Bothrops genus (10). Three years later, Manuel Uribe Ángel informed about the use of gold chloride as a neutralization method of snake venom, which was a method developed by Calmette for being used against European vipers (11, 12). However, it was only in 1896 that Evaristo Garcia published his work entitled “Los ofidios venenosos del Cauca”. The book described the anatomophysiological characteristics of snakes, the clinic manifestations of snakebite -including an excellent critique of traditional methods for their treatment-, and also new species known today as Bothrops punctatus (13). This book constituted the most important and complete work during that period, and it displays the scientific rigor of its time.
At the beginning of the twentieth century, Posada-Arango published his work entitled “Estudios científicos”, a significant portion of which was devoted to snakes and their venoms, traditional methods to treat snakebites -especially the use of plants, and Calmette´s serum-, and immunity (14). The monks Nicéforo María, Apolinar María, and Daniel Julián González,, with the collaboration of foreign researchers, conducted one of the most important studies about the snakes of our country. They published information about the classification of snakes in Colombia, the Boidae family, and coral snakes (15-17). In 1968, Medem published a work in which he described the advances of herpetology in Colombia, he informed about the distribution of reptiles throughout the country and included a comprehensive list of genera and species (18). Additionally, doctors Rodrigo Angel and Juan Silva Haad have also contributed to enriching our knowledge about the snakes of our country and snakebites. These authors have published information about general characteristics of snakes, venom composition, clinical alterations in snakebite patients and their treatment, and have also described new species (19-22).
During the last two decades, Drs. Carlos Alberto Cañas, Fernando Castro, and Rafael Santiago Castaño have worked in subjects including snakes, their venoms, and snakebite. A compilation of some of their work has been published recently, and deals with the evolution of snakes, species from Colombia, venom composition, and snakebites and their treatment (23). Finally, one of the most important contributions to snakebite, its epidemiology, and treatment in recent times has been made by the Ophidism/Scorpionism Program [Programa de Ofidismo/Escorpionismo] of Universidad de Antioquia. Nonetheless, the specific findings of this group will be discussed separately.
Ophidism/Scorpionism Program
The Ophidism/Scorpionism research group was created by the end of 1986 on the initiative of pediatrician Rafael Otero Patiño. One year later, Colciencias and Universidad de Antioquia approved a project related to the study of snakebites and snake venoms. Then, on March 1st, 1988, the program began its operation.
The 1990s.
During the last decade of the 20th century, cooperation between the Ophidism/Scorpionism Program and the Instituto Clodomiro Picado in Costa Rica, and different groups in Brazil was crucial. Several research projects carried out by Colombian researchers took place in laboratories from Costa Rica and Brazil. Besides, Costa Rican and Brazilian researchers were usually present in the Ophidism/Scorpionism Program’s publications related to the characterization of venoms and clinical trials. Indeed, their presence occurred in 70% of the Program’s publications during that decade. The above mentioned, once again, confirms that the research work of emerging groups gains in strength from the relationships that they establish with experienced researchers and groups in their area of action.
The first work produced by the Program involving Colombian snake venoms and their toxins was carried out by Lomonte et al. (24), and one of its co-authors was Dr. Rafael Otero. In that work, a basic phospholipase A2 (PLA2), from Bothrops asper venom -which at the time was taxonomically classified as B. atrox- was isolated and characterized. In 1992, the first work carried out exclusively by authors belonging to the Ophidism/Scorpionism Program was reported (25). In that work, several snake venoms from different regions of Colombia -focusing on the departments of Antioquia and Chocó- were characterized. Additionally, during the same year, the first works related to the clinical and epidemiological characteristics, as well as primary attention, of snakebite were produced (26, 27) Once again, those works focused on the departments of Antioquia and Chocó. Since its beginnings, the Ophidism/Scorpionism Program was constituted as a multidisciplinary group of professionals, including physicians, pharmaceutical chemists, veterinarians, zoologists, bacteriologists, biologists, among other professionals. Today, food engineers and biological engineers also make part of the program. Additionally, the Program offers graduate-level training, including master and Ph.D. degrees.
In 1995, Otero et al. (28) published a study about the ability of six antivenoms from Colombia, Mexico, and Brazil to neutralize B. asper venom. In this study, significant differences were observed between antivenoms, thus determining that the best treatment was the polyvalent antivenom produced by Instituto Nacional de Salud in Colombia. A similar study was conducted by Otero et al. (29), comparing a new monovalent antivenom against B. asper and two other commercial antivenoms. The new monovalent antivenom was produced by using an immunizing mixture of B. asper venoms from the departments of Antioquia and Chocó. The main conclusion of that study was that the new antivenom should be effective in treating snakebites that occurred in the above-mentioned departments of Colombia. Likewise, in the nineties, Otero et al. carried out several clinical trials with different types of antivenoms. Thus, in 1996, Otero et al. compared the antivenoms produce at Instituto Clodomiro Picado and a monovalent against B. asper venom (30). Both antivenoms were effective in neutralizing the biological effects provoked by snakebites. In 1998, Otero et al. performed a randomized, blinded, comparative clinical trial of one pepsindigested IgG and two whole IgGs (31). In that study, it was concluded that the efficacy of the three antivenoms was similar in neutralizing human B. asper envenoming and that the production of whole IgG antivenoms by caprylic acid fractionation is a good alternative for reducing the incidence of early adverse reactions (EARs). The last clinical trial performed during the nineties was presented by Otero et al. in 1999 (32). In that study, two whole IgG antivenoms were compared, one was prepared by means of caprylic acid fractionation, and the other was produced by means of ammonium sulfate precipitation. The study also included Porthidium nasutum snakebites.
All of these preclinical and clinical trials have been useful in order to optimize the treatment of snakebites in Colombia, including the number of antivenom vials necessary to neutralize toxins, the presence of EARs to antivenoms, and the neutralizing ability of imported products, among others. By the end of the 1990s, the Ophidism/Scorpionism Program also conducted an essential toxinology study related to Lachesis sp venom. In this work, venoms obtained from bushmasters from Costa Rica, Brazil, and Colombia were compared, thus detecting some similarities and differences. Venoms from L. stenophrys from Costa Rica and L. muta from Brazil had the highest lethal and hemorrhagic activities, whereas all tested venoms had similar electrophoretic patterns (33). In 1996 and 1999, Colciencias and Universidad de Antioquia sponsored two research projects related to ophidians from a specific sub-region of the departments of Antioquia and Chocó, and to plants used in traditional medicine to treat snakebites. Those projects led to the creation of one of the most active research lines of the Ophidism/Scorpionism Program, namely the “Food and therapeutic alternatives” research line. They allowed the program to produce the first papers of the 2000 decade.
The 2000s.
As mentioned above, the ethnobotanical study carried out in Antioquia and Chocó resulted in the identification of 101 plant species used by traditional healers. Different preparation methods were also determined according to the species and the part of the plant being used.
Thus, some plants are used in the form of infusions, others are prepared through decoction or maceration, and others are employed as external baths on the affected extremity. Under the guidance of healers, researchers collected 77 of such species (7). The second stage of the study was devoted to testing 74 plant species against the lethal effect of B. asper venom (classified as B. atrox in 2000). Seven species were identified as the most promising since they completely inhibited the lethal effect of B. asper venom when their extracts and venom were preincubated at 37 ºC for 30 minutes. Those species and the parts of the plants that were employed were as follows: stem barks of Brownea rosademonte (Caesalpiniaceae) and Tabebuia rosea (Bignoniaceae); rhizomes of Renealmia alpinia (Zingiberaceae) and Heliconia curtispatha (Heliconiaceae); whole plants of Pleopeltis percussa (Polypodiaceae) and Trichomanes elegans (Hymenophyllaceae); and the ripe fruits of Citrus limon (Rutaceae). The other five plant species partially neutralized the lethal effect of the B. asper venom (34). Finally, those plant extracts were tested against the hemorrhagic effect induced by B. asper venom, 31 out of 75 plant extracts had a moderate or high inhibitory ability. At the same time 12 of them demonstrated 100% neutralizing capacity when they were preincubated with venom as described above and then intradermally injected into mice (35). The most promising plant species were tested by their capability to inhibit the edema-forming and defibrinating activities induced by B. asper venom. Also, another test was conducted, such as their ability to neutralize other snake venoms, like L. muta, Crotalus durissus cumanensis, and Micrurus mipartitus (36, 37). Following those studies, some plants were chosen to be the subjects of future research. The most promising species were: Heliconia curtispatha, Renealmia alpinia, Piper peltatum, Brownea rosademonte and Bixa orellana. Along these lines, Núñez et al. (38) isolated an active compound from P. peltatum and P. umbellatum, 4-nerolidylcatechol, which inhibited the PLA2 activity of different venoms and reduced the myotoxic and edema-forming activities induced by B. asper myotoxins, when this compound was preincubated with toxins. Likewise, Pereañez et al. (39) demonstrated that other species of Heliconia sp., such as H. latispatha and H. wagneriana inhibited enzymatic activities of B. asper in vitro. Similar findings were obtained by Estrada et al. (40) for H. psitacorum and H. rostrata.
In 2001, Otero et al. (41, 42) discussed the actual situation of snakebite in the departments of Antioquia and Chocó They analyzed the real need for antivenoms in Colombia, concluding that snakebite was still a serious public health issue in our country, and that Colombia had a shortage of antivenom production, which continues to be the case today. In the 2000s, the Ophidism/Scorpionism Program continued conducting clinical trials. Otero et al. (43) informed about clinical complications in patients that suffered snakebites by snakes of the Bothrops, Porthidium, or Bothriechis genera. In that study, the complications identified included: acute renal failure, soft-tissue infection, central nervous system hemorrhage, compartment syndrome, soft-tissue hematomas, and placental abruption. Additionally, the study also concluded that the clinical pathophysiology of snakebites was similar to the ones previously reported. Besides, Otero et al. (44) tested the efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without beta-propiolactone, in the treatment of B. asper bites. The main conclusions obtained in that work were that both antivenoms had similar capacities to neutralized venom toxic effects and that both of them induced (mild) early adverse reactions at the same rate.
In 2003, the first study about the ontogenic variability of B. atrox and B. asper venoms from Colombia was carried out by Saldarriaga et al. (45). They reported variability in the composition of venom from specimens of B. asper and B. atrox of several ages (0, 6 months and 1, 2, and 3 years), being the venoms produced by newborns and juvenile specimens more lethal and hemorrhagic than the ones produced by adult snakes of both species.
In the 2000s, the Ophidism/Scorpionism Program began to study the ecological behavior of scorpions, as well as the clinical manifestations, treatments, and epidemiology of scorpion stings. Researchers reported some findings related to venom composition and the mechanism of action of venoms from some species. In a clinic-epidemiological study conducted in the departments of Antioquia and Tolima, Tityus pachyurus, Centruroides gracilis, T. fuehrmanni, T. asthenes and Chactas spp. were identified as the species that provoked the highest number of stings, which mainly occurred on hands and feet. Systemic signs of envenoming included vomiting, tachypnea, hypertension (in some cases), and acute edematous pancreatitis, in a three-year-old child (46). These findings were supported by the epidemiological analysis performed by Gómez and Otero (47). Along these lines, Barona et al. (48) characterized the composition of the most toxic and important scorpion venom from Colombia, T. pachyurus. They tested the capacity of three Latin American antivenoms (from Brazil, Mexico, and Venezuela) to neutralized the toxic effects of the venom. The study allowed them to determine that antivenoms from Mexico and Brazil neutralized the T. pachyurus venom. Finally, in the same year, Barona et al. (49) carried out a proteomic analysis of the venom from the species mentioned above, separating 57 fractions and identifying some of them. The study showed that some of those fractions have modulating activities on K+ or Na+ channels.
The other two studies involving scorpions were the ones performed by Gómez et al. (50, 51) with T. asthenes and T. fuerhmanni. Those works concluded that these species are less toxic than T. pachyurus; however, scorpion stings inflicted by the former require special attention in children.
2010 until today
During the 2010s, the Ophidism/Scorpionism Program started the study of venoms in a very minimalistic way, by isolating toxins, studying their mode of action, performing proteomic analysis and characterizing venoms that had never been studied before. Thus, Pereañez et al. (52) isolated a PLA2 from crotoxin complex from C. d. cumanensis, which is the toxin responsible for neurotoxicity in Colombian rattlesnake bites. This PLA2 has served as an important model for finding PLA2 inhibitors. Moreover, Patiño et al. (53) isolated two serine proteinases from venom mentioned above and informed about their anticoagulant activity. In the same year, Vargas et al. (54) cloned and characterized an L-amino acid oxidase from this venom, which had antimicrobial activity. Similarly, Patiño et al. (55) isolated and characterized a hemorrhagic snake venom metalloproteinase (SVMP) from B. atrox venom, which had biological activities comparable to BaP1, a SVMP from B. asper venom. This toxin, named Batx-I, has been used by the research group as an important model to search for SVMP inhibitors. Moreover, Vargas et al. (56) isolated an L-amino acid oxidase from Bothriechis schlegelii, which did not have cytotoxic activity nevertheless showed antimicrobial activity, similar to that isolated from C. d. cumanensis.
In 2014, Pereañez et al. (57) particularly in tropical and subtropical countries of Africa, Asia, Latin America and Oceania. Snake venoms are complex mixtures of toxic enzymes and proteins, where the most important and abundant muscledamaging components in snake venoms are phospholipases A2 (PLA2s informed about the isolation of a basic PLA2 from B. asper venom, which had myotoxic, edema-forming, cytotoxic, and anticoagulant activities. Posada et al. (58) isolated an acidic PLA2 from the same venom, which also induced myotoxic, edema-forming, cytotoxic, and anticoagulant activities. However, it is imperative to highlight that not all acidic PLA2s are toxic; hence, this was the first acidic PLA2 isolated from Colombian B. asper venom with toxic activities.
The “Food and Therapeutic Alternatives” research line of the Ophidism/Scorpionism Program has carried out several studies in order to explore new strategies to improve the treatment of local tissue damage induced by snake venoms. In that sense, several researchers have studied natural and synthetic compounds to inhibit the toxic effects of PLA2s and SVMPs. Pereañez et al. (59, 60) reported the potential inhibition of bile acids and phenolic compounds from plants to decrease the enzymatic and toxic effects of a PLA2. In 2012, Henao Castañeda et al. (61) synthesized substituted thiobenzoic acid S-benzyl esters and reported their capacity to inhibit the enzymatic activity of a snake venom PLA2. Employing a computational approach, the authors proposed that these synthetic compounds may bind to the PLA2-active site through H-bonds. However, these compounds have solubility drawbacks; hence, Henao Castañeda et al. (62) synthesized new thioesters, which were derived from 2-sulfenyl ethyl acetate, thus achieving a change in phenyl group for an ethyl ester group. The new compounds inhibited the PLA2-enzymatic activity with lower IC50 values than those described in 2012. Also, these compounds did not inhibit SVMPs significantly. The Ophidism/Scorpionism Program has also studied the potential of plant phenolic compounds to inhibit snake venom toxins. In 2011, Pereañez et al. (60) tested caffeic acid, ferulic acid, propylgallate, gallic acid, tannic acid, and epigallocatechin gallate, and demonstrated that these compounds inhibited PLA2-enzymatic and cytotoxic activities. Using molecular docking and a study of physicochemical properties, the authors proposed that these compounds may interact with the active site of the enzyme. Another phenolic compound tested against a snake venom PLA2 was biflavonoid morelloflavone, which inhibited the enzymatic, myotoxic, and edema-forming activities induced by the enzyme. By using the bioinformatic tool, molecular docking, and fluorescence, the authors proposed that the inhibitor may interact with the enzyme, specifically at the active site (63). Another tested compound was glycolic acid (64) (2013); however, it was assayed against the metalloproteinase Batx-I. This compound inhibited enzymatic, hemorrhagic, and edemaforming activities. Also, the authors suggested that glycolic acid chelated the cofactor Zn2+ employing fluorescence studies, chelation assays, and molecular docking. Other natural products tested against the same SVMP were triterpenic acids (65, 66), which inhibited the enzymatic activity of the enzyme, and they were characterized as competitive inhibitors. Moreover, these compounds inhibited the hemorrhagic, myotoxic, and edema-forming activities provoked by this toxin. Similar findings were obtained with the flavonoid myricetin (67). Nevertheless, it is important to mention that the latter studies included a new bioinformatic approach to describe the interaction between the inhibitor and the protein. This strategy was molecular dynamics and allowed for the calculation of the binding free energy of the interaction and revealed the atomic interactions that underlie binding between inhibitors and the toxin.
As mentioned above, the Ophidism/Scorpionism Program has used bioinformatic tools to describe some aspects of the mode of interaction between toxin inhibitors and snake venom proteins. Nonetheless, the research group has also applied this approach for predicting the molecular structure of toxins and its implications in biological and enzymatic activities, as it was the case of the crotoxin complex from C. d. cumanensis (68). Molecular modeling and molecular docking were also used by Pereañez et al. (69) to propose differences between hemorrhagic and non-hemorrhagic PI-SVMPs, suggesting that the former may form catalytic complexes with type IV collagen, laminin and perlecan of the basement membrane. In contrast, non-hemorrhagic ones may not form such catalytic complexes. These differences may explain the disparities in the biological activities of these metalloproteinases.
One of the most promising plants with antiophidic properties reported by the Ophidism/ Scorpionism Program is Renealmia alpinia, which was propagated in vitro in 2008 by Alarcón et al. (70). In addition, several studies have been carried out on this plant. As mentioned above, R. alpinia inhibited hemorrhagic, lethal, edema-forming, and coagulant activities of B. asper venom (34, 36, 71). In this context, Patiño et al. (72) informed the inhibitory capacity of wild and micropropagated extracts of R. alpinia on the hemorrhagic, edemaforming, fibrinogenolytic, desfibrinogenating, and proteolytic activities of a SVMP and a snake venom serine proteinase. Likewise, Patiño et al. (73) used an innovative methodology, which consisted of the oral administration of wild and propagated R. alpinia in vitro extracts before venom injection. R. alpinia extracts inhibited the lethal effect of B. asper venom and partially neutralized the venom-induced hemorrhage in the lungs, kidney, and hearth. In addition, Gómez-Betancur et al . (74, 75) isolated a flavone named Pinostrobin from R. alpinia, and tested its capacity to inhibit the whole venom of B. asper and an isolated PLA2. These authors reported that the Pinostrobin inhibited proteolytic, indirect hemolytic, and hemorrhagic activities of the whole venom and the compound also decreased the enzymatic, myotoxic, anticoagulant and edemaforming activities provoked by the PLA2. Moreover, the 2014 study informed about the analgesic activity of the R. alpinia extract. This aspect was studied in greater detail in 2015 by Gómez-Betancur et al. (76). These authors fractionated the dichlorometane extract of the aerial parts of wild and in-vitropropagated R. alpinia, and isolated some compounds, which had antinociceptive effects. Similar findings were reported by Gómez-Betancur et al. (2019) for the essential oils of wild and in-vitro-propagated R. alpinia (77).
Another promising plant is Swietenia macrophylla, which was chosen after a screening based on antiPLA2 and antioxidant activities. S. macrophylla showed a positive correlation between its inhibitory capacity on PLA2 activity and its antioxidant activity, as well as total phenol content (78). Thus, an activity-guided fractionation of an ethanol-soluble extract of the leaves of S. macrophylla resulted in some promising fractions that inhibited, in a dose-dependent manner, the cytotoxicity, myotoxicity and edema induction of PLA2s isolated from C. d. cumanensis and B. atrox (79). In recent studies, Preciado et al. (80) identified (+)-catechin as one of the active compounds in the most active fraction of S. macrophylla. At the same time, Henao et al. (81) evidenced the lack of oral toxicity of the same fraction at 300 mg/kg doses in mice in a fourteen-day study. Finally, Posada et al. (82) used histopathological analysis and reported the capacity of catechin and S. macrophylla fractions to inhibit myonecrosis, leukocyte infiltration, and promote collagen deposition. All of these findings suggest that S. macrophylla and its compounds are excellent potential sources of antiophidic molecules.
During the last decade, the Ophidism/ Scorpionism Program also conducted ethnobotanical studies that have contributed to existing ones (7). In 2013, one such study was carried out in the Eastern sub-region of the department of Antioquia. Twenty-nine species of plants were collected and identified, 82.7% of which were native. Out of the whole population, 27.5% of the plants had not been previously reported as antiophidian, while 38% of them had been employed for this purpose in other geographical areas. Some species did not have any ethnobotanical report; however, others, such as R. alpinia, whose antiophidic activity had been studied before, were also informed (83). In 2015, a similar study took place in the sub-regions of the department of Antioquia that had not been included in the previous research -namely: Magdalena Medio, Bajo Cauca, north, northeast, and southwest-. Seventy-one plant species were identified and collected, 49.29% of them did not have any previous reports as antiophidian, and 38.0% had been employed for the same purpose in other geographical areas (84). Some species have been reported in previous studies involving a different sub-regions of the department of Antioquia, which indicates that ancestral knowledge transmission did not have geographical barriers.
The Ophidism/Scorpionism Program has also used proteomic tools to study some snake venoms. For example, Rey-Suárez described venom composition in the most important coral snakes from Colombia, namely: Micrurus mipartitus and Micrurus dumerilii (85, 86) a proteomic strategy to determine their composition. Proteins were separated by RP-HPLC, followed by SDS-PAGE, in-gel tryptic digestion, identification by MALDI or ESI tandem mass spectrometry, and assignment to known protein families by similarity. These analyses were complemented with a characterization of venom activities in vitro and in vivo. Proteins belonging to seven families were found in Colombian M. mipartitus venom, including abundant three-finger toxins (3FTx; ~60% of total proteins. M. mipartitus had a substantial quantity of three-finger toxins (3FTxs) and a low percentage of PLA2s. In contrast, M. dumerilii had a high percentage of PLA2s and low proportion of 3FTxs. Both venoms induced neurotoxic symptoms in mice. Moreover, these results have supported the hypothesis describe by Lomonte et al. (87), which establishes a dichotomy of Micrurus sp. venom phenotypes regarding the relative abundance of the omnipresent PLA2s and 3FTxs: a group of species expresses a PLA2- predominant venom composition, while others display a 3FTx-predominant compositional pattern. The PLA2-predominant venoms are not neutralized by antivenoms produced by using 3FTx-predominant Micrurus sp. venoms, and vice versa. Proteomic studies with coral snake venoms were the starting point to research their most toxic components. Rey-Suarez et al. (88) characterized the most abundant protein of M. mipartitus; a 3FTx named Mipartoxin-I, which was lethal in mice and blocked the post-synaptic receptor of acetylcholine, thus being neurotoxic. On the other hand, Rey-Suarez et al. (89) have been purified and characterized from coral snake (Micrurus spp. have also characterized two PLA2s from M. mipartitus (MmipPLA2) and M. dumerilii (MdumPLA2). The first one was lethal and myotoxic in mice, while the second one was non-lethal but myotoxic in mice. Also, Rey-Suarez et al. (90) characterized an L-aminoacid oxidase from M. mipartitus (MmipLAAO), which is a nonlethal protein, but it has antimicrobial activity. Proteomics was also used by Fernández et al. (91) to analyze and characterize the venom of semiarboreal viper B. punctatus. This work allowed Toxinologists, who had little knowledge about this species and its venom, to learn that the venom of this snake has a good correlation between venom composition and biological activities and that the most abundant group of toxins in B. punctatus venom were SVMPs (41.5%), in a similar way to other bothropic snake venoms. In order to inform about venom composition and to compare it with venoms from other geographical locations and their neutralization by antivenoms, similar proteomic analyses were performed with L. acrochorda, B. atrox and C. d. cumanensis (92-94).
In recent years, the Ophidism/Scorpionism Program has also studied the biology of spider and scorpion venoms. Along these lines, researchers from the group participated in the description of two new species of spiders: Pamphobeteus verdolaga and Aguapanela arvi (95, 96). Likewise, a transcriptomic analysis of the last one species was performed by Estrada et al. (97), evidencing the presence of some common toxins and some uncommon proteins in Theraphosidae venoms. Other studies with spider venoms included partial characterization of the venoms from Phoneutria boliviensis and Pamphobeteus nigricolor (98, 99), demonstrating that these venoms have PLA2 enzymatic activity and have toxins that could affect ion channels. Likewise, scorpion venoms of medical importance from Africa and Colombia have been characterized. Those venoms include the ones from five different scorpions of the Buthidae family -Androctonus amoreuxi, Babycurus jacksoni, Grosphus grandidieri, Hottentotta gentili and Tityus fuhrmanni-, and one of the Scorpionidae family -Pandinus imperator-. Some of these venoms showed PLA2, proteolytic, and cytotoxic activities (100) (Estrada-Gómez et al., 2017). Additionally, other scorpion venoms from Colombian scorpions have been characterized, as is the case of Ophisthacanthus elatus and Cetruriodes edwarsii (101, 102). All of these works have improved our knowledge about arthropod venoms from the species mentioned above. They have provided us with important information in order to understand the clinical basis of the scorpion and spider stings. In addition, the venoms of scorpions and spiders from Colombia have a great potential for research about bioactive molecules, such as antimicrobial, larvicidal and antitumoral ones.
Other researches
On the one hand, at Universidad del Valle and Fundación Valle del Lilli, researchers have contributed to our knowledge about clinical manifestations of M. mipartitus and B. punctatus snakebites by informing about some clinical cases inflicted by these species (103, 104). Likewise, Cañas reported a case of brainstem ischemic stroke after a B. atrox snakebite, evidencing the incidence and importance of cardiovascular complications of bothropic snakebite in Colombia (105). Similarly, Jimenez-Charris et al. (106-108) from Universidad del Valle as well, conducted a proteomic a study of P. lansbergii lansbergii from the department of Atlántico, where the activities of acidic and basic PLA2s were tested, and the antitumoral activity of a particular acidic-PLA2 activity was evaluated. These works have enriched our knowledge about P. lansbergii lansbergii, which had not been studied before.
Also, researchers from Universidad del Cauca have carried out a proteomic study about the venom of B. ayerbei, which was considered a variation of B. asper. However, in 2010 it was classified as a new species. In that study, important differences between B. ayerbei and B. asper were detected. For example, the venom of the first one has a negligible content of PLA2s, which goes in contrast with the higher concentration of those toxins in the B. asper venom (109). In contrast, Guerrero-Vargas et al. (110) identified novel Na+ channel toxins in the venom of T. pachyurus. Furthermore, researchers from the same university isolated an antimicrobial peptide from the poison of the frog Dendropsophus colombianus (111). Dr. Santiago Ayerbe has been an active researcher on clinical toxinology and biology/ ecology of snakes. In his works, this researcher has described the clinic basis, epidemiology, and treatment of snakebite in the department of Cauca (112, 113). In addition, Ayerbe et al. (114) have described new Micrurus species.
Other important advances have been made by the group led by Camila Rengifo, which has described the neuromuscular activity of M. dissoleucus and M. mipartitus venoms, evidencing a postsynaptic action of M. mipartitus venom and a partial blockade by the venom of M. dissoleucus (115). Neuromuscular effects from adult and juvenile specimens of C. d. cumanensis were also studied, showing that both venoms have the same activity, but the venom from adults was faster at producing a neuromuscular blockade than the one from juveniles (116). Moreover, this group published a protocol for obtaining toxin sequences from venoms employing purification of mRNA, which is an excellent tool to avoid mRNA extraction directly from animal glands (117).
Finally, other important findings from Colombian toxinologists have been obtained at Universidad de Los Andes, where venom from Colombian Latrodectus sp was characterized for the first time (118). That study demonstrated that the venom of Colombian Latrodectus sp induced effects that were similar to those of other venoms from this genus and contained proteins of different molecular masses. In addition, the PLA2 catalytic differences of venoms from B. asper and C. d. cumanensis were also studied at Universidad de Los Andes, showing that both venoms prefer anionic substrates, but B. asper venom had slightly lower activity than rattlesnake venom (119).
CONCLUSIONS
The toxinologic work in Colombia has contributed to our knowledge about endemic species, and clinical manifestations of snakebites and scorpion stings. However, we invite Colciencias and other funding agencies to assign more resources to support a higher number of researchers in this field, since snakebite is a neglected public disease and needs more attention from governments and scholars. Finally, we still lack much information about the venoms of some species and their possible mode of action. In addition, given the complexity of venoms, we ignore the potential use of toxins in current biomedicine. Thus, many more studies in toxinology need to be made.