INTRODUCTION
Foodborne diseases (FBD) are a major public health concern and a significant cause of morbidity due to the high incidence in the world. According to the World Health Organization (WHO) (1), FBD affects individuals of all ages, particularly children (under five years) and people living in low-income regions. It is estimated that each year, nearly 600 million people get sick by eating contaminated food, and 420,000 dies from the same cause. In Colombia, in 2015, a total of 10,243 cases of FBDs and waterborne diseases were reported to the National Public Health Surveillance System (SIVIGILA) (2), of which 1,434 cases were registered in the Antioquia department, being Medellin the city with the most significant number of reported cases (3).
Protein-rich foods like milk are more susceptible to microbial contamination. Therefore, it is necessary to evaluate its microbiological quality as well as its derivatives products. Traditionally crafted Soft Cheese is an essential part of the Antioquian basic basket, with a production of 1,608,914 kg/year (4). The Antioquian Soft Cheese is a fatty cheese of high humidity (aw of 0.98) and relatively elevated pH. It is consumed fresh and is made from whole or semi-skimmed sanitized cow’s milk (5). This cheese has a shelf life of approximately one week and must be kept at refrigeration temperature (6). Its physicochemical characteristics are ideal for the growth of altering microorganisms and especially pathogens such as Listeria monocytogenes, Escherichia coli, Salmonella, and Staphylococcus aureus (6). It is an extremely perishable product that, in some cases, when is elaborated in an artisan way, cannot fulfill all the hygienic and sanitary requirements for consumption, increasing the risk of microbial contamination (7).
Traditional food-preservation techniques and chemical preservatives have been extensively used to control the growth of food spoilage microorganisms (8). However, synthetic preservatives have been associated with allergic reactions, degenerative diseases, even certain types of cancer (9, 10). As an alternative, the use of microorganisms such as Lactic acid bacteria (LAB) and their antimicrobial metabolites is increasing not only to limit the growth of pathogens and food spoilage but also to improving their shelf life, nutritional, and sensory characteristics (11, 12).
Bacteriocins are a family of antimicrobial peptides produced by bacteria through ribosomal synthesis, are active on strains closely related to the producer strain, and sometimes to strains across genera (13). May acts as helper peptides of probiotics strains in the gastrointestinal tract (GI) (14), and some of them have been used as food preservatives, added as purified or partially purified concentrates in pork, fermented milk, cheese, milk, sausage, among other foods (11, 15-17).
Bacteriocins are majorly classified into three classes due to their genetic, biochemistry, and structural diversity (18). The class I, are bacteriocins containing unnatural amino acids inserted as posttranslational modifications. Nisin represents this class, a lantibiotic classified with “generally regarded as safe” (GRAS) status for particular applications by the Food and Drug Administrations (FDA) (19). Class II is the largest and structurally diverse group of bacteriocins, characterized by their high thermostability at broad pH range. This class contains small (<10 kDa) non-modified peptides, cationics, and highly hydrophobic. Class II is subdivided into class IIa pediocin-like bacteriocins, which share a highly conserved hydrophilic and charged N-terminal part harboring the consensus sequence -YGNGV- and a more variable hydrophobic and amphiphilic C-terminal part. Class IIb two-peptides unmodified bacteriocins, Class IIc circular bacteriocins, and Class IId unmodified, linear, non-pediocin-like bacteriocins. As opposed to Class II, Class III bacteriocins are large, heat-labile structures. Bacteriolytic enzymes as Enterolysin A produced by Lactobacillus crispatus Colicin produced by Escherichia coli belong to this group, as well as non-lytic proteins such as Caseicin 80 and Helveticin J (20).
Bacteriocin identification has been carried out through classical bioprospecting, which involves isolating and purifying the compound for its subsequent characterization and validation of biological activity (21). The costs and time necessary for this process, added to the legal limitations associated with access to genetic resources, allow us to consider the use of in silico methods that have been developed in recent decades as an alternative.
Genome mining involves predicting, identifying, and recognizing gene patterns or groups of biosynthetic gene clusters (BGCs), using tools from computational biology and information technology. In this way, it is possible to extract and process the information present in the sequenced genome of an organism, to identify genes that produce a protein of interest (22, 23).
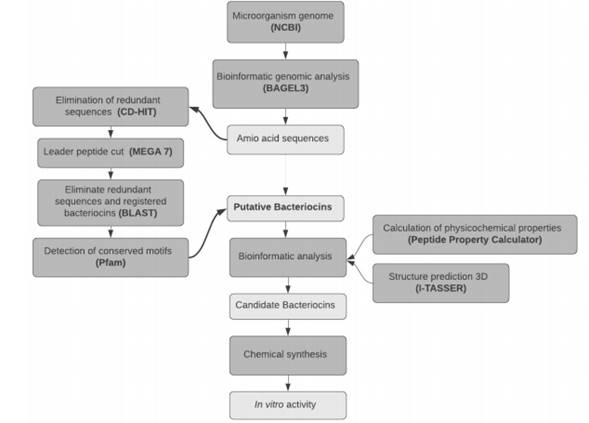
Two specialized databases allow predicting putative bacteriocins. The BACTIBASE (24) stores bacteriocins and enables users to predict them using a hidden profile of Markov models, applying as input the primary peptide sequence. The second database, BAGEL3 is specialized in predict putative bacteriocins in bacterial genomes. Using DNA sequences as input instead of annotated genomes, BAGEL discovers new bacteriocins through detecting structural genes and genes associated with bacteriocin production (25) which is largely independent of open reading frame (ORF. The purpose of this article is to evaluate the effect of a bacteriocin found by in silico methods on the microbiota present in Antioquian soft cheese.
MATERIALS AND METHODS
Bateriocin identification
Screening of reference genome for bacteriocins gene clusters
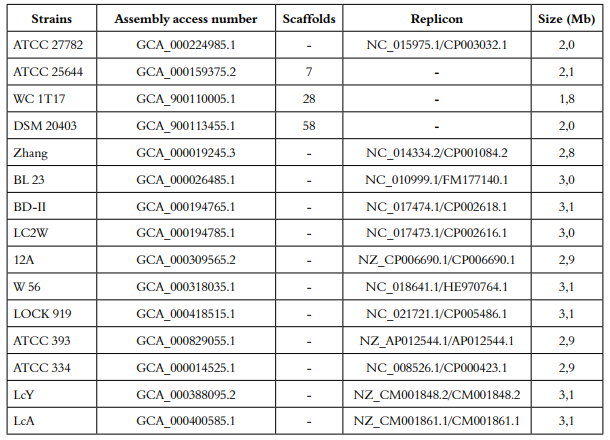
Draft genomes of eleven strains of Lactobacillus casei available in NCBI genomic database (http://www.ncbi.nlm.nih.gov/genome/) (January - June 2016), were screened for putative bacteriocins gene clusters using the web-version of BAGEL3 (http://bagel2.molgenrug.nl/index.php/bagel3). Accession numbers (Table S1) and screening identification workflow are described in the additional (File S1).
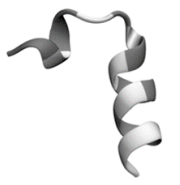
The three-dimensional (3D) structure of putative bacteriocin Bac22 was determined using the I-TASSER server and physicochemical characteristic was calculated with Peptide Property Calculator (http://www.genscript.com).
Preparation of peptides
Bac 22 was sy nthesized by Biomatik (Wilmington, DE, USA), lot number P171107- DG241345, manufactured on November 16, 2017 with a purity level the 83 %.
Standard solutions of Bac 22 (1000 μM) were prepared in sterile distilled water, considering the molecular weight of the peptide and its purity. This stock was used to prepare diluted solutions with a final concentration of 100 μM. A commercial preparation of nisin (Nisaplin®, Danisco, Grindsted, Denmark), which contains 200 IU/mL of nisin was prepared following the instruction of manufacturer. This concentration was chosen based on previous tests (data not shown).
Hemolytic activity
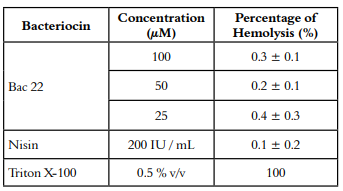
Hemolytic activity of BAC22 was tested upon human erythrocytes (red blood cells RBCs) from a healthy donor. Erythrocytes were separated by centrifugation at 1,000 g for 7 minutes. The supernatant was discarded, the pellet washed three times and resuspended in 10 mL of sterile saline solution. Briefly, the test consisted of mixing 90 μL of the erythrocyte suspension and 10 μL of Bac 22 at concentrations of 100, 50, and 25 μM, or Nisin at 200 IU/mL. As a negative control (NC), 10 μL of saline solution was added to 90 μL of erythrocyte suspension, and as a positive control (PC), Triton X-100 at 0.5 % v/v. The test was carried out by triplicate. The samples were incubated at 37 ° C for 3 hours with constant agitation and then centrifuged at 1,000 g for 7 minutes. After centrifugation, the supernatant was used to determine the amount of hemoglobin released. Fifty μL of the supernatant of each treatment was added to a well in a 96-well plate, and the absorbance of samples at 545 nm recorded using a microplate reader (ThermoFisher Scientific, USA). The hemolysis percentage for each sample was calculated using Equation 1.
(Equation 1: Percentage of Hemolysis (H %)
Matrix evaluation
The antibacterial activity of Bac 22 bacteriocin was evaluated against the microorganisms of the Antioquian soft cheese, a domestic product from Antioquia, Colombia.
Production of Antioquian soft cheese
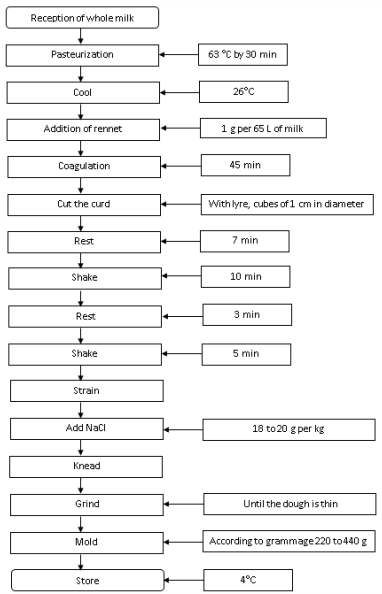
Fresh refrigerated raw milk from Paysandú Agrarian Station, a village of Santa Elena, Department of Antioquia, Colombia, was used plus curdling agent and common salt. Soft cheese was made at the Dairy Products Plant of Universidad Nacional de Colombia at Medellin. In Figure 1, the elaboration process of soft cheese is described.
The obtained soft cheese was evaluated under different microbiological tests, such as mesophiles counts, coagulase-positive S. aureus, total coliforms, and fecal coliforms; all of them, according to the Colombian Technical Standard NTC 5894. The purpose was to compare the effect of bacteriocins on the microbiological quality of soft cheese, when the peptide was added.
Microbiological analysis
Soft cheese (1 g) was used for the microbiological analysis. The treatment consisted of adding to the soft cheese, 100 μL (100 μM) of Bac 22, or 100 μL (200 IU/mL) of Nisin, or 100 μL of sterile water as a negative control. Two experimental units of each batch were taken for conducting the microbiological analyzes.
Each packed soft cheese plus the added compound was stored for approximately eight days under controlled conditions at 4 ºC. Microbiological sampling was carried out during the storage time following a partially staggered design, starting with an initial sampling at zero time (day zero), day one, day two, the middle of the trial (day four), and at the end of the experiment (day eight).
UFC/g count of mesophiles
One gram of soft cheese was added to 9 mL of sterile 0.1 % p/v peptonized water; it was stirred in a Stomacher (Seward, UK) for 1:30 minutes; next, the mix was serially diluted in peptonized water 0.1 % w/v until 10-4. A volume of 1 mL of each dilution was inoculated in a sterile Petri dishes in duplicate, and then covered it with 12 to 15 mL of Plate Count agar (Merck, Darmastadt, Germany), they were blended by rotation in an eight movement and waited until solidification. The Petri dishes were incubated at 35 °C for 48 hours.
UFC / g count of total and fecal coliforms
The same procedure, as described before, was followed; however, the dilutions were made up to 10-3 using Chromocoult agar (Merck, Darmastadt, Germany) and the bacterial cultures were incubated at 35 °C for 24 hours.
UFC / g count of coagulase-positive Staphylococcus aureus
The same procedure, as described before, was followed. However, from a single dilution, 200 µL were taken and inoculated on the surface of Baird Paker agar (Merck, Darmastadt, Germany) in Petri dishes by exhaustion, finally, the petri dishes were incubated at 35 °C for 24 hours.
RESULTS
Bacteriocin identification
Eight new putative bacteriocins of L. casei were identified from its genome using genomic mining (data not shown). Bac22 is a bacteriocin class II produced by L. casei 12A, which has a short sequence of non-modified amino acids (DSIRDVSPTFNKIRRWFV), a molecular weight of 2,237 kDa, an isoelectric point of 11.38 and charge of +2. Figure 2 shows the three-dimensional structure of the putative bacteriocin Bac22.
DISCUSSIONS
Bacteriocin Identification
Bac22 is a bacteriocin class II produced by L. casei 12A and was identified using bioinformatics tools. Singh and cols (26), using the bioinformatic tool BAGEL 3, classified fifty-two putative bacteriocins across 20 LAB species, that have not been previously reported as bacteriocins producers, and class II was the most predominant. Similarly, Alkhalili and cols (27) analyzed 252 genomes and identified seven putative novel class-I lanthipeptides using BAGEL 3. Oliveira and cols (28) predicted in silico (using BAGEL 3) a putative bacteriocin produced by L. rhamnosus L156.4, and they confirmed the bacteriocin inhibition growth of several bacteria in vitro, including gram-positive human and foodborne bacterial pathogens.
The putative Bacteriocin Bac 22 was selected for in vitro assay due to their high similarity with the bacteriocin m2386 previously reported by Kuo y cols (29) active against several Listeria species, and because their short sequence facilitates and reduces chemical synthesis costs.
Hemolytic activity
In the Table 1 Bac 22 showed percentages of hemolysis of less than 1 % in all its concentrations, including the maximum concentration evaluated (100 μM), similar Nisin, which is a bacteriocin for commercial use.
In other studies, carried out with purified bacteriocins of Enterococcus faecalis, no percentages of hemolysis were reported at concentrations of 2 μg/mL or less, Nisin at 10 μg/mL reported 1.1 % hemolysis in sheep erythrocytes (30). Similarly, in the bacteriocin Aureocin A70 partially purified to a concentration of 1,024 AU/mL was observed less than 1% hemolytic activity against sheep red blood (31). These results indicate that the Bac 22 bacteriocin designed in this research does not present hemolysis against human cells, which makes it a peptide with a possible commercial utility.
Food matrix evaluation
In Colombia, few studies have determined the microbiota of Antioquian soft cheese and how it relates to its preservation. Some authors indicate that combining the effects of bacteriocins with refrigeration, mesophilic aerobic count in white cheeses can be reduced, thus, obtaining a longer shelf life (32).
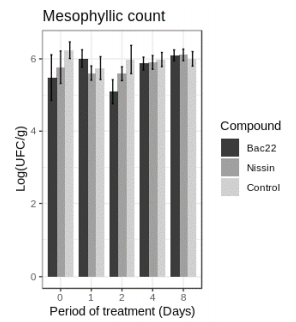
When evaluating the antibacterial activity of bacteriocin (Figure 3), both Bac 22 and Nisin, on the count of mesophiles in Antioquian soft cheese, did not show significant differences. Where Bac 22 significantly reduced the count of colonies compared to control on days zero and two (p 0.05) in the treatment between Nisin and Bac 22, except for days 2 and 4, where Bac 22 presented a decrease in the total coliforms count. Regarding the control, Bac 22 shows a significant reduction (p <0.05). Castro et al., 2009 (33) reported the effect of Nisin on CFU / g count of aerobic mesophiles with a decrease of approximately two magnitude orders (2 logs) in white cheese samples compared to control throughout the treatment. In our case, control was maintained in a stable amount of mesophiles.
In the last days of the test, the mesophil count (Figure 3) does not show differences between the treatments with both bacteriocins and control. It is even observed that the number of colonies is a little smaller compared to time zero. It is known that microbiota present in cheeses, especially LAB, can affect the number of microorganisms in a food system. This kind of bacteria can produce metabolites such as organic acids, like lactic acid, and bioactive peptides like bacteriocins, which lower the pH, and also, they compete for nutrients (34).
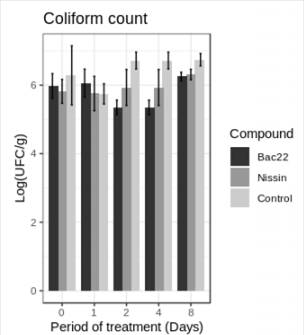
Similarly, the total coliform count, no significant differences were observed (p> 0.05) in the treatment between Nisin and Bac 22, except for days 2 and 4, where Bac 22 presented a decrease in the total coliforms count. Regarding the control, Bac 22 shows a significant reduction (p> 0.05) in the treatment between Nisin and Bac 22, except for days 2 and 4, where Bac 22 presented a decrease in the total coliforms count. Regarding the control, Bac 22 shows a significant reduction (p< 0.05) in the last days of the test (Figure 4).
Nisin is a bacteriocin that acts mainly over Grampositive bacteria, although previous studies show its effect on some Gram-negative microorganisms such as E. coli (14,35). Castro et al., 2009 (33), obtained the lowest count of coliforms with Nisin added to pasteurized milk before the white cheese elaboration process, indicating that Nisin was more efficient than the use of starter cultures. In the case of Antioquian soft cheese Bac 22, there was a more significant decrease in total coliforms during the treatment.
The microorganism L. casei produces the bacteriocin Bac 22, which is part of the microbiota from various dairy products (36). It is possible that due to this, it has a better effect on the microbiota of Antioquian soft cheese. However, investigations are necessary to validate this idea because, to date, no study evaluates the microbiota of Antioquian soft cheese and its effect on shelf life.
It should be noted that, in most evaluations of bacteriocins on cheeses, these peptides are added to the pasteurized milk before the coagulation process (38-40). In the present study, bacteriocins were added to the final product due to the small amount of Bac 22 and the large production volumes of the Dairy Plant of Universidad Nacional at Medellín. Future investigations using Bac 22 may be designed considering more available material, which could be added to the cheese before the coagulation process, hoping to obtain promising results like those of this research, and that can be compared with other works.
In all treatments and controls, for the detection of coagulase-positive S. aureus and fecal coliform were reported absent during the test, possibly due to good manufacturing practices during the elaboration process of soft cheese, such as good cleaning and disinfection of the equipment.
CONCLUSIONS
Bac 22 had minimum hemolysis percentages, less than 1%, similar to Nisin, demonstrating that, despite being a synthetic bacteriocin from LAB, it is safe for human consumption. Bac 22 showed a significant reduction over CFU/g of total coliforms concerning Nisin during study time, indicating that it could be a good alternative to be used in the elaboration of Antioquian soft cheese for better shelf life. Additionally, this bioactive peptide could be a conservation alternative for other types of food matrices, including those that contain a high number of total coliforms.