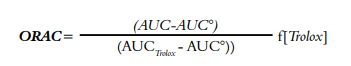INTRODUCTION
Oxidative stress has been associated with the development of chronic diseases, including colorectal cancer CRC, cardiovascular diseases, neurological disorders, and inflammatory diseases (1). This process is caused by a shift in the balance between free radicals such as reactive oxygen species (ROS) and antioxidant species towards free radicals, which may react with cellular macromolecules (1).
ROS can attack the lipids of the membrane, mainly polyunsaturated fatty acids (PUFAs), leading to the formation of hydroperoxides and aldehydes(2). Among the most studied aldehydes are malondialdehyde and 4-hydroxynonenal (4-HNE), which form DNA adducts (2). These species can also induce oxidative damage in DNA, including apurinic/apyrimidinic sites, oxidative modification of purines and pyrimidines, and strand breaks (3). 8-oxo-7,8-dihydroguanine is one of the best-studied oxidative stress biomarkers, and several studies have demonstrated a direct correlation between 8-oxodG formation and carcinogenesis in vivo(4).
It has been shown that regular consumption of fruit, rich in antioxidants could help to counteract oxidative stress and improve antioxidant status in vivo (5-6). These antioxidants or phytochemicals such as phenolic acids, flavonoids, and carotenoids are known to be able to sequester free radicals and positively regulate the endogenous antioxidant system of the cells, including tripeptide glutathione (L-γ-glutamyl-L-cysteinyl-L- glycine) (7-9). Glutathione is a cofactor for several antioxidant enzymes, and it can be present within the cells as the oxidized or reduced form (10).
Mango (Mangifera indica) is a tropical fruit widely consumed worldwide and is one of the fruits which has shown antioxidant activity (11). This activity has been attributed to its content of ascorbic acid, carotenoids, and polyphenols, among them mangiferin, one of its most studied components (12-13). It is a glucosylxanthone (2-b-D-glucopyranosyl-1,3,6,7-tetrahydroxy-9Hxanthen- 9-one), which is more abundant in the leaves and bark of Mangifera indica L (Anacardiaceae) and it can also be found in about sixteen plant families (14-15).
Mangiferin has demonstrated antioxidant capacity in different in vitro studies, including a study in which it showed the ability to sequester DPPH and ABTS radicals (13). It has also shown a protective effect against oxidative stress in vivo in Wistar rats subjected to iron overload, where induced an increase in the activity of superoxide dismutase and glutathione peroxidase compared to the control group (16). There are more than a thousand cultivars of mango, including “Azúcar” which is grown in Colombia and exported to several countries (17); it has shown antioxidant capacity in vitro, measured by the DPPH technique (17).
Therefore, this study aimed to determine the effect of mango juice consumption (cv. Azúcar) on the antioxidant capacity of plasma and biomarkers of oxidative stress in healthy individuals with low consumption of vegetables and fruit.
MATERIALS AND METHODS
Reagents
Fluorescein, 2,2´ - azino - bis (3 - ethylbenzthia zoline - 6 - sulfonic acid (ABTS•+), gallic acid, 6-hydroxy-2,5,7,8-tetrame-thylchroman-2- carboxylic acid (Trolox), 2-thiobarbituric acid, malondialdehyde and mangiferin were purchased from Sigma- Aldrich (St. Louis, Missouri, USA). Ethanol, n-butanol, acetic acid, Folin-Ciocalteu reagent, sodium carbonate, and trichloroacetic acid (analytical grade) were purchased from Merck (Darmstadt, Germany).
Juice and placebo preparation
Ripe mango (cv. Azúcar) fruits were purchased from Medellin (Colombia) market and immersed in a sodium hypochlorite solution (100 ppm), washed with water, peeled, sliced into small pieces. The juice was made homogenizing those pieces in a blender with water (1:4). The final total solids were measured with a digital refractometer, and the results were expressed as Brix degrees at 20°C. Finally, the juice was pasteurized at 85°C for 10 min, sweetened with 0.5 g/l of commercial sucralose, packaged into low-density polyethylene containers and immediately stored at 4°C. Xanthan gum was used as a stabilizer (1 g/l). pH and acidity (%) of the juice were determined. A food engineer designed the placebo drink, and it was similar in terms of taste and color to the mango juice, but it did not have phytochemicals. Placebo was developed mixing mango flavor (2.5 g/l, Bellchem), red colorant (10 mg/l, Bellchem-Colombia), commercial saccharose (60 g/l), fructose (40 g/l, BellchemColombia), titanium dioxide (180 mg/l, BellchemColombia), water, carboxymethylcellulose (4.5 g/l, Bellchem-Colombia) and xanthan gum (1 g/l).
Microbiological analysis
Count of Escherichia coli was carried out by the most-Probable-Number (MPN) method, according to the Food and Drug Administration’s Bacteriological Analytical Manual (18). Aerobic mesophilic bacteria, yeasts, and molds were counted by agar plaque technique, and the results were expressed as a colony-forming unit (CFU).
Subjects and study design
Women and men between the ages of 18 and 57 years old from “Universidad de Antioquia” (Medellin, Colombia) were recruited via advertisements. After administering a questionnaire concerning their dietary habits, potential participants were invited to participate in the study, and they provided written informed consent. Sixteen individuals who consumed ≤ 2 servings/ day of fruit or vegetables were selected. They did not meet the following exclusion criteria: 1) smoking; 2) take medication or supplemental vitamin and/or mineral; 3) suffer from any inflammatory bowel disease, cancer, diabetes, gastrointestinal, renal or infectious diseases; 4) pregnancy; 5) vegetarian diet; 6) to consume more than 2 fruit or vegetables/day.
The individuals participated in 73 days, crossover and, single-blind (to the participants) study with two intervention periods (Figure 1). Volunteers were randomized to receive 200 mL/day either mango juice or placebo for 26 days in the morning; each participant involved in juice and placebo was spaced by a 3-week wash-out period. Throughout the study, subjects had to avoid the consumption of foods rich in carotenoids. Therefore, a list of vegetables and fruit which the subjects were not allowed to eat was provided: (fruit: watermelon, tangerine, cantaloupe; vegetables: spinach, broccoli, chard, sweet potato, carrot, cabbage, red pepper, and lettuce). Participants were asked not to consume mango or mango derived-products except for the one provided during the study.
Anthropometric assessments (height and weight) were measured. The body mass index (BMI) was calculated as body weight kg/height (m)2 and defined as normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obesit y (>30 kg/m2 ) according to OMS guidelines(19).
Food logs were kept by the subjects during the intervention periods to check their compliance with the dietary restriction. No information on quantitative food consumption was available because the diaries were food records by menu without any information on quantities.
The research protocol was approved by the Ethics Committee of odontology faculty at Universidad de Antioquia (Act number 5, August 2014).
Blood sampling
At the end of the placebo and juice period, fasting (10h) venous blood samples (10 mL) were collected from each volunteer in tubes containing sodium heparin. Each whole blood sample was centrifuged for 15 min at 3500 rpm. Plasma was carefully separated into microcentrifuge tubes and stored at −80°C until use. Erythrocytes were mixed with 5% metaphosphoric acid in water (w/v), stored on ice for 10 min and centrifuged at 12000 rpm for 10 minutes at 4°C. Finally, the supernatant was collected and stored at −80°C.
Determination of total phenolic content
The total phenolic content was determined according to a modified Folin-Ciocalteu (20). Plasma (10 μl), 125 μl of Folin-Ciocalteu reagent, and 400 μl of sodium carbonate solution (7.1% w/v) were mixed, the resulting solution was incubated at room temperature for 30 min in the dark. Gallic acid was used as a reference standard. The absorbance was measured at 760 nm against a blank, and the results were expressed as mg of gallic acid equivalents (GAE)/l.
ORAC (Oxygen Radical Absorbance Capacity) assay
The assay was performed by mixing 21 μl of fluorescein solution (10 Μm), 2899 μl of 75 mM phosphate buffer (pH 7.4), 50 μl of 600 mM AAPH (2,2’-Azobis(2-amidinopropane) dihydrochloride) and 30 μl of plasma. Fluorescence was measured on a Perkin Elmer® LS55 spectrofluorometer with a thermostatted multicell. The results were expressed as μM Trolox equivalent antioxidant capacity (TEAC)/l, according to the following Equation 1:
AUC is the area under the curve of the sample, AUC° is the area under the curve for the control, AUCTrolox is the area under the curve for Trolox and f is the dilution factor for juice (20).
ABTS•+ assay
Plasma (10 μL) and stock solution ABTS•+ (990 μl) were mixed, and the resulting solution was incubated at room temperature for 30 min in the dark. The absorbance was measured at 734 nm against a blank. Trolox was used as a reference standard, and the results were expressed as μM Trolox/l(20).
Determination of mangiferin
Mangiferin was determined to confirm that all participants took the juice when it was provided. Identification and quantification of mangiferin were carried out using Shimadzu® Prominence (LC-20AD) HPLC system. The flow rate was 0.6 ml/min, and the injection volume was 10 μl. Separation of mangiferin was carried out using the Lichrospher® RP C18 column (5µm, 250 mm x 4 mm) at 30°C. The mobile phases were 2% (v/v) acetic acid (A) and (B) 5% acetic acid in water and acetonitrile (50:50, v/v). The gradient elution was as follows: 5% B (0-1 min), 5-25% B (2-10 min), 25-55% B (10-40 min), 55-90% B (40-45 min), 90-55% B (45-50 min), 55-5% B (50-55 min), 5% B (55-60 min). Mangiferin was determined at 258 nm only once in plasma samples from individuals after mango juice consumption (21).
Thiobarbituric acid reactive substances (TBARS)
Lipid peroxides, including MDA, can react with TBA to form a colored complex that can be measured at an excitation wavelength of 500 nm and an emission wavelength of 520 nm(22). Five hundred microliters of plasma, 80 μl of trichloroacetic acid (1%), and 160 μl of thiobarbituric acid (6%) were mixed and incubated for 20 min at 90°C. The reaction mixture was immersed in cold water for 10 min, and 600 μl of butanol was added to it. MDA was used as a reference standard, and results were expressed as µmol of malondialdehyde/l of plasma. Fluorescence was measured on PerkinElmer® LS-55 spectrofluorometer (Perkin-Elmer, Beaconstield, U.K.).
Determination of GSH (glutathione) total
The level of total GSH was measured using a colorimetric ELISA kit (catalog number STA-312; Cell-Biolabs®, San Diego, CA, USA) in lysed erythrocytes according to the manufacturer’s instruction. The absorbance was measured at 405 nm every 1 minute for 10 minutes. Results were expressed as µmol/l.
Determination of DNA damage
The levels of 8-hydroxydeoxyguanosine (RNA) and 8-hydroxy-2’-deoxyguanosine (DNA) were measured using a colorimetric ELISA kit (catalog number 589320; Cayman Chemicals, Michigan, USA) on plasma samples according to the manufacturer’s instruction. The absorbance was measured at 412 nm and the results were expressed as pg/ml.
Statistical analysis
The sample size was calculated using Statmate Graphad, and it had an 80% power to detect a difference between group mean and the hypothetical mean of 0.36 with a significance level (alpha) of 0.05 (two-tailed). The statistical power was acceptable, according to the literature (23).
Assays were conducted by triplicate, and data were reported as the mean ± standard deviation (SD). Paired T-student estimated the differences between groups, and a value of p < 0.05 was considered statistically significant. The results were analyzed using SAS university.
RESULTS
Microbiological and physicochemical analysis
The count of both mesophilic bacteria and E. coli were 500 and < 10 CFU in the juice. The count of yeast and molds were < 200 CFU in the juice (data not shown).
The total acidity, pH, and °Brix of mango juice in the day of its preparation were 0.32%, 3.8 and, 8, respectively. The juice had 18% of mango fruit. Placebo showed 9.7 °Brix.
Baseline characteristics and anthropometrics
Only sixteen participants completed the study, of which thirteen were women, and three were men; eleven had a normal BMI (19). The BMI was measured to verify that juice consumption did not affect corporal weight taking into account that fructose has a lipogenic effect (24). Participants met the dietary restriction and took the placebo or juice as we recommended during the study.
Total phenolic content
It was evaluated total phenolic content in plasma, but it did not show significant differences between juice (1759.2 ± 275.0 GAE/l) and placebo (1792.5 ± 293.1 GAE/l) period.
Antioxidant activity
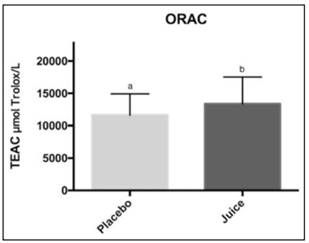
Antioxidant activity in plasma was measured by ABTS•+ and ORAC methods (Figures 2 and 3). ABTS value was higher after juice consumption (4492.3 ± 496.5 µmol Trolox/L) compare to placebo (4153.0 ± 539.7 µmol Trolox/L), and they showed significant differences between them (p < 0.05). Also, antioxidant activity measured by the ORAC method was higher after juice consumption (13183.9 ± 4080.7 µmol Trolox/L) compared to placebo (11760.4 ± 3215 µmol Trolox/L) and they showed significant differences between them (p < 0.05).
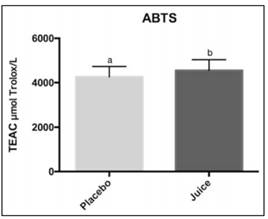
Figure 2 Effect of mango juice consumption on plasma antioxidant capacity measured by the ABTS•+ method. Values are the means ± standard deviation of three replicates. ABTS: (2,2´-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid).
Mangiferin on plasma
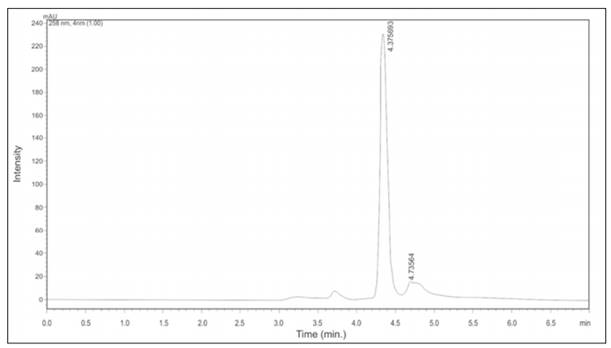
Mangiferin is one of the characteristic components of mango; then, it was determined by HPLC in plasma samples after (Figure 4) and before juice consumption. Mangiferin was within a range of 7.05 ± 0.02 to 28.9 ± 0.87 mg/l after juice consumption, and the mean concentration was 14.4 ± 9.6 mg/l; it was also not detected before juice consumption (data not shown). The retention time of mangiferin was 4.3 min.
Mangiferin was measured on plasma samples at the end of the juice consumption by HPLC at 258 nm.
Effect of mango juice on lipid peroxidation
TBARS assay was used to detect MDA in plasma as a marker of lipid peroxidation. TBARS value was similar after juice consumption compared to placebo (Table 1), and they did not show significant differences between them (p > 0.05).
Values are the means ± standard deviation of three replicates (n=16). Paired t-test. TBA: thiobarbituric acid.
Effect of mango juice on GSH total levels
Table 1 shows total GSH levels on plasma samples. GSH total did not show significant differences after juice consumption compared to placebo (p > 0.05).
Effect of mango juice on DNA oxidative damage
Table 1 shows 8-OhdG levels in plasma samples. The 8-OhdG levels did not show significant differences after juice consumption compared to placebo (p > 0.05).
DISCUSSION
Low consumption of vegetables and fruit has been associated with a higher risk of chronic diseases, including colorectal cancer; however, their action mechanisms have not been fully elucidated (1, 25). It has been suggested that the antioxidant activity of vegetables and fruit components have an important role in the prevention of these diseases (5). This activity could help to prevent oxidative stress, such as lipid peroxidation and DNA damage in vivo (1).
Several studies have shown that individuals with low consumption of vegetables and fruits have higher levels of lipid peroxidation and DNA oxidative damage (26, 27). This evidence indicates that those foods protect perhaps against oxidative stress and have chemopreventive potential (5).
MDA is a lipid peroxidation product that may modulate some cell functions, including cell proliferation, and it also can react with DNA bases forming adducts, which could be mutagenic; therefore, contributing to the cancer development including CRC (28).
Dietary consumption of polyphenols has shown a direct association with plasma antioxidant capacity. This has been evidenced by Wang et al. (2012), demonstrating that an increase in the consumption of foods rich in polyphenols increased the plasma antioxidant capacity which is inversely correlated with oxidative stress (29). However, total phenols did not show significant changes, and this could be due to their low concentration in plasma after 20 hours of juice consumption. These compounds have shown a maximum concentration observed (Cmax) of ≤ 1 µM and time of maximum concentration observed (Tmax) between two and four hours (Cmax), then at the time of blood sampling, their concentration should have been very low (30). Therefore, it would be interesting to evaluate the phenolic compounds’ level using more sensitive techniques such as HPLC-MS (30).
This study showed that mango juice improved plasma antioxidant capacity, which is in agreement with previous studies. Robles-Sánchez et al. (2011) evaluated the effect of whole and fresh-cut mango (cv. Ataulfo) consumption for 30 days on plasma antioxidant capacity by the ORAC and the ABTS methods. They found that mango improves plasma antioxidant capacity in healthy individuals compared to control. Whole and fresh-cut mango showed ORAC values corresponding to 460 ± 0.14 and 370 ± 0.13 μM Trolox equivalent, respectively, and they showed ABTS values of 730 ± 0.08 and 800 ± 0.10 μM Trolox equivalent, respectively (31). These values are lower compared with our results, which indicate the higher antioxidant capacity of mango cv. Azúcar compared to cv. Ataulfo.
ABTS values of placebo period were higher than those reported by previous studies, which showed values corresponding to 1,310 and 675 µmol Trolox/L in healthy individuals. These results may be due to differences in the levels of antioxidants from the participants’ diet and other molecules present in the plasma with antioxidant activity such as albumin, ceruloplasmin, and uric acid (9, 32).
Vece et al. (2015) evaluated the association between CRC risk and dietary total antioxidant capacity (TAC) by TEAC assay (Trolox Equivalent Antioxidant Capacity) in an epic cohort with 45,194 individuals. They observed that individuals in the highest quintile of TAC, compared with those in lowest, had a statistically significant lower risk of colon cancer (relative risk: 0.63; CI: 0.44-0.89) (33).
Pardo-Andreu et al. (2006) evaluated the effect of mango stem bark extract (Vimang) consumption on plasma antioxidant capacity of elderly subjects for 30 days. They observed an increase in plasma antioxidant capacity compared to baseline value measured by ABTS radical, corresponding to 1390 ± 0.057 μM Trolox equivalent (9). However, Vimang is different from our juice because the former has a high concentration of mangiferin, a xanthonoid which has shown antioxidant activity comparable to the activity of vitamin C (9, 21).
To our knowledge, mangiferin was detected for the first time after mango juice consumption on plasma samples. This achievement is remarkable because it has shown to be able to increase the activity of enzymes such as superoxide dismutase, catalase, and glutathione peroxidase in the liver and kidney of streptozotocin-induced diabetic rats (34). It would be important to do further studies to evaluate the effect of mango juice consumption on those enzymes.
The maximum plasma concentration reported after a single oral administration of mangiferin (0.9 g) to healthy adults was 38.64 ± 6.75 ng/ml (14). This value is lower than our values, which could be explained by the accumulation of mangiferin due to daily juice consumption; however, further studies would be necessary to confirm this.
TBARS levels didn’t show significant differences after mango juice consumption compared to placebo. However, Pardo-Andreu et al. (2006) found a positive effect of Vimang on lipid peroxidation in elderly subjects; they found lower values of plasma TBARS levels compared to the baseline values after 30 days of extract consumption corresponding to 14.67 ± 0.742 µmol/l (9). They also determined the effect of Vimang in total GSH values, and they didn’t observe any change in total GSH values after extract consumption, which is consistent with our results. Nevertheless, we cannot ensure if mango may influence this tripeptide because we did not measure oxidized GSH levels, which are high during oxidative stress (9).
Several studies have observed a direct correlation between 8-oxodG formation and carcinogenesis in vivo (4), it was evaluated if mango juice consumption affects this biomarker. Still, it did not show significant differences after juice consumption compared to placebo.
This study had some weaknesses: first, we didn’t collect information related to the food type and quantity consumed daily by each participant to know what other foods could have been involved in the antioxidant activity on plasma. We only had the information about each participant’s food logs. Second, mango composition has shown to be different within samples and influenced by harvest date, weather, and location (12), so it is not possible to fully control batch to batch variation of the mango samples. Therefore, in this study, mangoes were bought from the same supplier for both intervention periods to minimize variation within samples. Third, although some participants drop out of the research for several reasons such as change of worksite and trips, the power of the sample size was recalculated, and it was of 80%.
Finally, although blood sampling wasn’t done at the beginning of each intervention period, the placebo allowed a baseline comparison for the juice supply period. BMI values weren’t normal in every participant, but this probably didn’t influence plasma antioxidant capacity according to a previous study that observed no significant differences in total antioxidant capacity between normal and overweight/obese individuals (35).
CONCLUSIONS
Regular consumption of mango (cv. Azúcar) juice improved plasma antioxidant capacity, but it didn’t influence oxidative stress biomarkers. Further studies would be necessary to evaluate the mango juice’s effect on the endogenous antioxidant system (superoxide dismutase, catalase, and glutathione peroxidase).