Introduction
Humankind has used plants as a medical resource for the traditional treatment of various diseases, attributing a wide variety of therapeutic properties against bacteria and fungi (1). The isolation and identification of biologically active compounds and molecules have led to discovering new compounds, helping improve medical therapies and applications in different industries (2). Different plant materials have been selected based on their common interactions between plants and the environment, considering that secondary metabolites benefit different human applications (3). Plants are an extraordinary source of compounds with biological activity, which permits us to take advantage of them to treat various diseases (4). An example of this plant richness is the Caesalpinia genus with more than 500 species, with different classes of chemical compounds isolated, such as flavonoids, diterpenes, and steroids (5). Reports indicate that several species of this genus exhibit a wide range of pharmacological properties, including antiulcer, anticancer, antidiabetic, anti-inflammatory, antimicrobial, and antirheumatic activities that could have potential in ethnomedicinal practices (6, 7, 8). Additionally, it is important to highlight that the richness of the products derived from Caesalpinia species are not only associated with pharmacological use but also agricultural industries (9), animal production (10), and environmental applications for the oil industry (11).
Group A beta-hemolytic Streptococcus pyogenes produce scarlet fever and strep throat. S. pyogenes are gram-positive cocci, which are non-motile round shape bacteria. This kind of microorganism synthesizes a toxin that causes a rash and a disease named streptococcal toxic shock-like syndrome. There is an overestimation of the bacterial source in throat infections, severe bronchitis, and S. pyogenes resistance to beta-lactam antibiotics. The fungus that most often causes cutaneous candidiasis is Candida albicans. C. albicans is a yeast-like microorganism that has been known to be the root cause of many recurrent and chronic diseases when they get out of equilibrium in the human organism, particularly in the vaginal tract, even though it can disturb the nail beds, mouth, and bloodstream (12, 13).
Due to inadequate consumption of antibiotics, an effect known as multiple drug resistance (MDR) is being generated in human medical services, which is a serious threat to public health; thus, antimicrobial compounds for the pharmaceutical industry are an important research issue (2, 14). Furthermore, the emergence of MDR among pathogenic microorganisms has limited the effectiveness of antibiotics, and this trend is a concern to agencies such as the World Health Organization. Faced with this problem, a search for bioactive compounds with antibiotic properties could be an alternative to MDR. In Colombia, specifically in the municipality of La Guajira, there is a wide probability of finding antimicrobial substances because of the presence of species of the genus Caesalpinia (15). Further, the Wayúu community that occupies this area uses fruit extracts of C. coriaria (Jacq.) Willd against different skin and mucous illnesses. Moreover, considering the MDR, it is necessary to search for new substances from unresearched C. coriaria with probably appealing performances, at least partially, on MDR pathogens including bacteria or fungi. Therefore, this study evaluated the antimicrobial activity of methanolic and ethanolic extracts of C. coriaria (Jacq.) Willd dry fruits on strains of S. pyogenes and C. albicans.
Materials and methods
Extraction and preparation of plant extract
The C. coriaria material was collected in the Toroqui community (which belongs to Wayuu community) in the rural area of the municipality of Riohacha, La Guajira (11˚29ʼ51.7 ”N & 072˚50ʼ03.0" W) approximately 142.91 m2, the area is classified as tropical dry forest (16). The plant sample consisted of 500 g of ripe fruits of C. coriaria, applying a standard technique (17). First, the fruits were dried in a shadow and crushed to pulverize. The subsequent powder was stored in amber glass flasks at an average temperature of 25ºC until processing. Then, 50 g of the pulverized material was taken to obtain the extract, and two extraction processes were realized as follows: (1) absolute methanol, (2) ethanol 98%, applying in all cases the Soxhlet technique (for 2 h and 48 h, using 200 mL of each solvent). Once the solvent had evaporated, a dry extract was obtained and stored aseptically under refrigeration at 4˚C in hermetic bottles.
Phytochemical tests
The plant extracts were subjected to screening to establish the presence of secondary metabolites through color tests as follows:
Shinoda´s test for flavonoids
In a test tube, 3 mL of 10% extract, one fragment of magnesium tape was placed, and 1 mL of concentrated HCl was added. After 5 minutes of reaction, 1mL of amyl alcohol was added. A yellow, orange, brown coloration indicates flavonoids.
Braemer´s test for tannins
0.3 g of dry extract was dissolved in 3-mL methanol, and 2 mL of 10% alcoholic ferric chloride solution was added. The test was considered positive for tannins when the sample took on a bluish-black or green.
Test for alkaloids
In two test tubes, 1-mL extract and 1-mL HCL were added to each one. Meyer's reagent was added to the first tube and Wagner's reagent to the second, the formation of a cream precipitate was scored positive for Mayer's test, and the appearance of a brown precipitate for Wagner's test for the presence of alkaloids.
Test for Glycosides
0.1g of dry extract was dissolved in 2-mL pyridine, then 2-mL sodium nitroprusside solution was added after it was made alkaline with 5% sodium hydroxide solution. The sample, which turns pink to red indicates glycosides.
Test Afrosymetrical
With 0.4g of the dry alcoholic extract in a test tube, 5 mL of distilled water was added. In a water bath, the solution was heated for 2 minutes and vigorously shaken. The test was considered positive for saponins when the foam persisted for 5 minutes or more (18).
Borntrager´s Test
To 10-mL benzene, 0.2 g of the alcoholic extract was added, which was stirred and filtered by gravity. 5 mL of 10% ammonium solution was added to this filtrate, and it was carefully stirred. The appearance of a pink, red, or violet in the ammoniacal (lower) phase was taken as the presence of free anthraquinones, according to Opinde and collaborators (19).
Antimicrobial activity
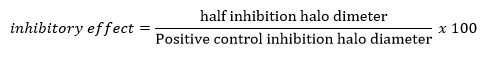
S. pyogenes ATCC 12384 and C. albicans ATTC 14053 were obtained from the Microbiology Laboratory of the Universidad Popular del Cesar (Valledupar, Colombia). The antimicrobial activity of the extracts was performed applying the implemented Kirby-Bauer method (17). Disks impregnated (6 mm) with plant extract were placed with the following concentrations: 2,100, 1,050, 525, and 262.5 mg/mL as a qualitative test, using a negative control (distilled water) and positive control (ampicillin 500 mg/10 mL for S. pyogenes and fluconazole 150 mg/mL for C. albicans), three replicates of each treatment were performed. They were incubated at 37ºC for 24h for S. pyogenes ATCC 12384 and 48 h for C. albicans ATTC 14053. After the incubation time, the presence of inhibition halos around the impregnated discs was determined. The diameter of each halo was measured in millimeters (mm) (20), and the calculation of the percentage of the inhibitory effect relative to the positive control, which was proceeded by applying the following equation (21):
Equation (1):
The presence or absence of an inhibition zone was used as a criterion to select the extract that best inhibited the growth of the pathogenic microorganisms understudied. Then, the disk diffusion test was performed, and Minimum Inhibitory Concentration (MIC) was determined on Mueller Hinton agar (S. pyogenes ATCC 12384) and BHI agar (C. albicans ATTC 14053) according to The Clinical and Laboratory Standards Institute (CLSI) using four filters Whatman paper discs (6 mm diameter) impregnated with different concentrations of the extract (262.5-152 mg/mL) in the same incubation conditions. Therefore, we can figure out our data only with different diameters of inhibition. Finally, the collected data were analyzed using the ANOVA test (R software v.4.0.2.).
Results
The principal aim of this study was to evaluate the antimicrobial effect of two extract types obtained from dry fruits of C. coriaria. First, the soxhlet method was conducted, getting different efficiencies between the methanol and ethanol extracts. Specifically, with methanol extract, a greater amount of dry extract was produced; the maximum value obtained was 14 g, and with ethanolic extract, a maximum value of 8.5 g was obtained; in this way, the methanol presented higher yield rates. Additionally, a different secondary metabolite was identified from each extract (Tab. 1). Finally, the level of the obtained metabolite from each extract was evaluated visually according to the expected color for each phytochemical test.
Table 1 Type of secondary metabolite identified from Soxhlet technique using 200 mL of methanol and ethanol as solvent.
| Metabolites | Extract | |
|---|---|---|
| Methanol | Ethanol | |
| Flavonoids | + | + |
| Tannins | ++ | + |
| Alkaloids | +++ | ++ |
| Glycosides | ++ | + |
| Saponins | + | ++ |
| Anthraquinones | +++ | ++ |
Low +, Medium ++, High +++
The current study supports previous findings in the literature that antimicrobial activities directly relate to increasing the concentration of the extracts. Significant antimicrobial effects of each C. coriaria extract on test microorganisms are given in Figures 1 and 2.
The ethanolic and methanolic extracts of C. coriaria showed antimicrobial activity on S. pyogenes ATCC 12384; the highest antimicrobial activity was recorded at 2,100 mg/mL (Fig. 2, p < 0,05), and representing the highest inhibition percentage (29.6 for methanolic extract and 28.4 for ethanolic extract). A two-way ANOVA was performed for those samples. The main differences were found for the concentration variable, suggesting that the two evaluated extracts had no differential antimicrobial effect.
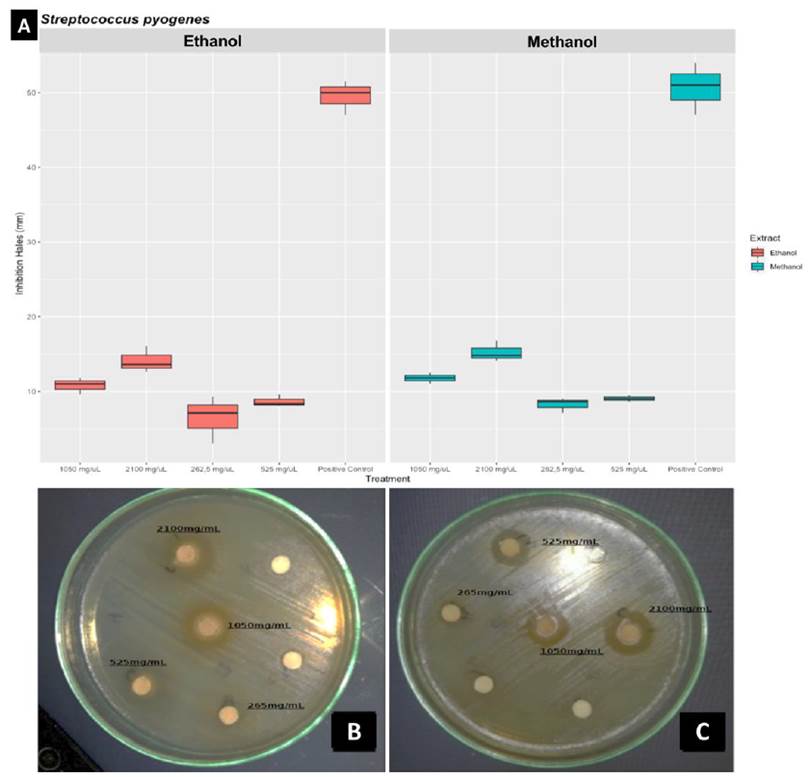
Figure 1 Antimicrobial evaluation of the methanolic and ethanolic extracts against S. pyogenes ATCC 12384. A: Boxplot of each extract and concentration. B and C Antibiogram, B: ethanol, C: methanol, disc without concentration mark were negative control (water).
Regarding the inhibitory effect on the strains of C. albicans ATTC 14053, only the ethanolic extract of C. coriaria showed antimicrobial activity, the highest response was obtained at a concentration of 2,100 mg/mL (Fig. 2), and its inhibition percentage was 50.7.
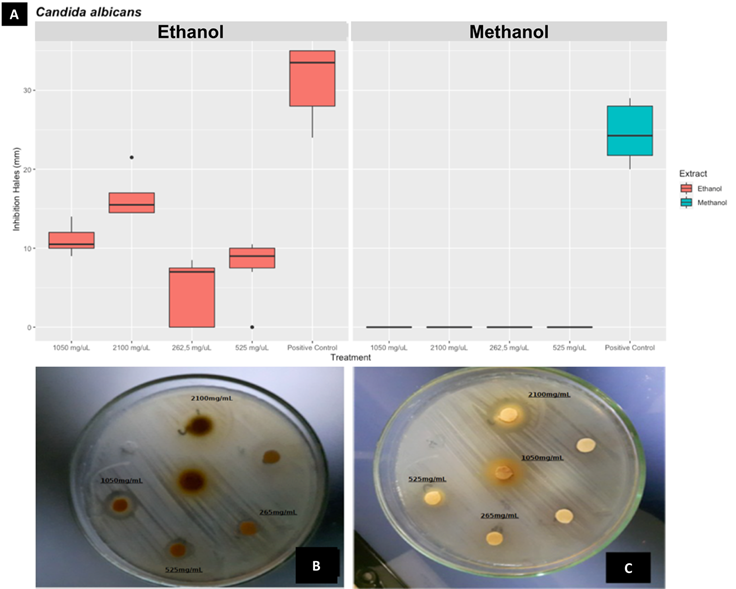
Figure 2 Antimicrobial evaluation of the extracts against C. albicans ATTC 14053. A. Boxplot for inhibition hales for different concentrations. B and C Antibiogram B: ethanolic extract and C: Methanolic extract, disc without concentration mark were negative control (water).
Finally, the C. coriaria extracts showed MIC against S. pyogenes ATCC 12384 at a concentration of 172 mg/mL; concerning the results of the growth of C. albicans ATTC 14053 against the different concentrations of ethanolic extract of C. coriaria, it was established that the MIC was 212 mg/mL.
Discussion
The ethnobotany and ethnopharmacology disciplines define "medicinal plant" as those used in traditional medicine containing beneficial elements in the cure of diseases in humans and / or animals (2). This work evaluated the antimicrobial activity of methanolic and ethanolic extracts of C. coriaria (Jacq.) Willd dry fruits on S. pyogenes ATCC 12384 and C. albicans ATTC 14053. Therefore, the extraction efficiency of the fruit extract was first evaluated with the Soxhlet method. The results showed a highly efficient process, in which the extraction performed using methanol produced a more significant amount of dry extract; for this reason, it was considered the solvent with the highest yield compared to ethanol, which presented a lower efficiency.
Among the solvents most used today for extracting natural substances of biotechnological interest are aliphatic alcohols (methanol and ethanol) (22), these organic solvents are efficient, and their use is simple due to their low toxicity for humans, also, considering it safe and efficient extraction procedure (23). Furthermore, the concentration of solvents and their mass/volume ratio significatively affect the extraction of metabolites due to their excellent capacity to extract both lipid and water-soluble substances, which produce different yields and extract substances such as alkaloids, flavonoids, glycosides, and terpenes, among others (24). Finally, it is also important to highlight that extraction efficiency does not directly correlate with the inhibitory activity in the bacterial and yeast species evaluated in this study.
Regarding C. coriaria, this species is distributed in the Colombian Caribbean region, mainly in Atlántico, Bolívar, Cesar, La Guajira, Magdalena, and Sucre. This species is known to have abundant tannins, hence its many uses, such as dye leather. Rural communities from La Guajira prepare water and rinses to relieve tonsillitis, both from cooked fruits and infused leaves used to treat diarrhea (25, 26). This diversity of uses has also been reported in other countries, such as Mexico (27). The Caesalpinia genus with more than 500 species emerges as an alternative for investigating pharmacological activity, where different chemical compounds such as flavonoids, diterpenes, and steroids have been isolated (5, 25).
In this study, employing a phytochemical analysis, it was possible to determine glycosides, steroids, phenolic alkaloids, tannins, saponins, quinones, flavonoids, anthraquinones; however, some highlighted substances, such as coumarins, were not found in methanolic extracts. These results are similar to those reported by Mohana (29) and Anandhi et al. (30), who also reported tannins, quinones, carbohydrates, saponins, flavonoids, glycosides, cardiac glycosides, terpenoids, phenols, coumarins, proteins, steroids, and anthraquinones. However, they could not determine the presence of alkaloids and triterpenes; these differences were possibly due to intrinsic and extrinsic factors such as soil type, origin, environmental temperature, cultivation method, harvest, or extraction.
For the Caesalpinia genus, it has been reported that methanol extracts have a greater inhibitory effect in different microorganisms (17, 31); for example, high inhibitory activity is reported with methanolic extract using C. nepalensis (32). In other studies, it was possible to verify the antimicrobial activity of C. ferrea Martius on S. mutans, S. salivarius, S. oralis, and Lactobacillus, finding high effectiveness in MIC (25, 40, 66, and 100 µg/mL) (30). Also, in ethanol-based extracts of C. mimosoides, they presented a MIC <1 mg/mL against bacterial and fungal strains (34, 35).
Soares et al. (36) reported the antimicrobial activity of C. ferrea extracts against the most common oral pathogenic bacteria and fungi such as C. albicans, S. mutans, S. salivarius, S. oralis, and L. casei, showing to be more effective against C. albicans, by generating inhibition hales of 15 mm in diameter, these results are similar to those obtained in this work.
Sharma et al. (38) demonstrated that the antimicrobial activity of C. decapetala extracts against fungal strains (Aspergillus fumigatus and C. albicans), Gram-positive bacteria (Staphylococcus aureus and S. pyogenes), and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) using the method dilution of the agar wells. The antibacterial effect presented by this plant species was attributed to phytoconstituents such as alkaloids, glycosides, phenols, phytosterols, saponins, and flavonoids, similar compounds found in this study.
Research by Glauber et al. (38), using other plant species, demonstrated antimicrobial activity of C. ferrea extracts against C. albicans, S. mutans, S. salivarius, S. oralis, and L. casei. They found inhibitory halos of 21mm, 19.5mm, 18.5mm, 12mm, 10mm, 11mm, 13mm through the disk diffusion method, which can be compared with the results obtained in our work with its intermediate effectiveness (see Figures 1 and 2).
Finally, this great variability of results from different investigations suggests that the inhibitory effects of secondary metabolites depend on plant species, plant tissue, and extraction method. Therefore, more studies must establish precisely the maximum efficiency and inhibitory effects for specific species of bacteria and fungi and establish a baseline with data on species, plant tissue, and extraction method for performance and specific pharmacological research.
Conclusion
The plant species studied in this research was shown to have significant activity against selected microorganisms. We could confirm various phytochemicals in the extracts, highlighting the antibacterial and antifungal activity of extract of C. coriaria. It was concluded that the ethanolic extract of C. coriaria had the highest antibacterial activity against S. pyogenes and C. albicans. The phytochemical analysis showed that the extracts contain different molecules, assuming that dry fruits of C. coriaria contain bioactive compounds of potential therapeutic and prophylactic importance, and therefore it is a promising organism for research.