INTRODUCTION
Ilex guayusa Loes (Guayusa) belongs to the Aquifoliaceae family; it is a native Amazonian shrub that reaches 6 to 10 m in height, found in countries such as Ecuador, Bolivia, Peru, and Colombia (1). It is distributed in the Amazon foothills, in the Andean mountains at an altitude of 200 to 2,000 m.a.s.l. (2).
Several studies have reported its traditional use such as rituals by the inhabitants of the Amazonian territory of Ecuador due to its stimulating and healing properties (3,4). Currently, Guayusa has gained relevance in Ecuador and in the world for its health-promoting properties and especially for its high caffeine content, which could explain the development of nutraceuticals, dietary supplements, and antioxidants (5). Guayusa leaves have been reported to be used by many Amazonian communities as a diuretic, diaphoretic, emetic, hypnotic, narcotic, stimulant, and purgative (6).
In recent years, an important number of studies have been conducted on the chemical composition of I. guayusa, considering this species as an interesting source of secondary metabolites, such as pentacyclic triterpenes, xanthines, flavonoids, phenolic compounds, carotenoids, saponins, and derivatives of chlorogenic acid (7-9).
The bioactivity of I. guayusa extracts could be strongly related to phenolic compounds and flavonoids (2). These compounds are well known because they have different biological activities, such as their high antioxidant activity, anti-inflammatory agents, and importance in inhibiting various pro- inflammatory mediators. They have been used to manage metabolic syndromes, cancers, cognitive disorders, and the immune system (10).
Due to these antecedents, in recent years, the interest in this type of compounds has increased since epidemiological studies reveal that the consumption of foods and beverages with a high content of phenolic compounds and flavonoids have been associated as protective agents against fatal degenerative diseases, such as cardiovascular disease, cancer, and diabetes, as they are closely related to oxidative stress produced by free radicals in the body (11). This has led many studies to focus on natural products that can contribute to the prevention and reduce the risk of suffering from diseases that are difficult to treat. Therefore, the objective of this study was to evaluate the antioxidant capacity and determine the total content of phenols and flavonoids from extracts obtained from the leaves of Ilex guayusa.
MATERIALS AND METHODS
Plant material
The plant samples, 5 kg of I. guayusa fresh leaves, were collected in “Mogambo Sendero Ambiental”, located in Viotá, Cundinamarca, Tequendama province, 90 km from Bogotá; 1310 m.a.s.l, and 20.5° C annual average temperature. It is a rain forest ecosystem located at the mountain foot, under the plus code (zip code) CG78+QW. Voucher number 30173 Herbarium of Pontificia Universidad Javeriana.
Extracts preparation
One kilogram of I. guayusa fresh leaves, dehydrated at room temperature, went through cold maceration with ethanol at 96% for 30 days to obtain the total ethanolic extract (TEE). Alternatively, 1 kg of leaves, dehydrated at room temperature, was extracted by soxhlet method for a week with increasing polarity solvents, initially with dichloromethane (ED), then ethyl acetate (EA), and finally ethanol (EE) to obtain increasing low-mid, mid-high, and high polarity extracts, respectively. The extracts were concentrated at reduced pressure in an IKA HB10 rotary evaporator and were dry stored in a refrigerator at 4°C. Based on obtained extracts, solutions were prepared at a 1,000-ppm concentration in methanol for antioxidant tests.
DPPH• Scavenging Capacity Assay
The antioxidant capacity of the extracts obtained from I. guayusa was evaluated by scavenging capacity of the radical DPPH• (2,2-diphenyl-1- picrilhydrazil), analytical grade, Sigma-Aldrich) according to Brand-Williams et al. (12) method with some modifications. 990 µL of a methanolic solution of DPPH• (0.1 mM) was mixed with 10 µL of the extracts at 40 to 200 ppm. The mixtures were kept at room temperature and in the darkness for 30 min. Afterward, the absorbance at 520 nm was measured in a spectrophotometer (Spectronic 21D, Milton Roy). Antioxidant capacity was quantified using ascorbic acid, Trolox, and Rutin calibration curves. Methanol was used as a negative control. Each measure had three replicates. The antioxidant capacity of extracts was calculated as I = [(AB − AA)/AB] × 100, where I is DPPH• inhibition (%); AB is the absorbance of a blank sample, and AA is the absorption of the extract solution.
ABTS•+ Scavenging Capacity Assay
It was carried out by the method proposed by Miller et al. (2) with some modifications. The radical was generated by an oxidation reaction of ABTS•+ ((2,2-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid)) with potassium persulfate (K2S2O8). The extracts were evaluated in a concentration range of 40 to 200 ppm, of which 20µL were taken and mixed with 980µL of the ABTS•+ solution previously prepared in a phosphate buffer solution at a pH of 7.4. The obtained solutions were incubated in the dark at room temperature for 30 min. Subsequently their absorbances at 734 nm were measured in a spectrophotometer (Spectronic 21D, Milton Roy).
An ascorbic acid, Trolox, and Rutin calibration curve were used. Methanol was used as a negative control. Each measure had three replicates. The percentage inhibition of ABTS •+ of the test samples was calculated according to the following formula:
% inhibition = [(AB − AA)/AB] × 100, where AB is the absorbance of a blank sample, and AA is the absorbance of the test sample.
Quantification of total phenols
The modified Folin-Ciocalteu (13,14) method was used for total phenols quantification. 100 µL of the extracts solution (5 mg/mL) were added to 200 µL of the Folin-Ciocalteu reagent (diluted 1:10) in semi-micro PMMA cells. After three minutes, 750 µL of Na2CO3 (7.35%) were added and allowed to react in the dark for 2 hours (determined by reaction kinetics). After the incubation, the absorbance at 735 nm was measured in a Thermo Scientific Evolution 60S Spectrophotometer. Each measurement was performed in duplicate. Content of total phenols was determined by interpolating the absorbance in a calibration curve previously constructed using gallic acid as a standard; the result is presented in mg Eq of Gallic Acid /g of Dry Extract (15).
Quantification of total flavonoids
Total flavonoid content was determined by the Aluminum chloride (AlCl3) modified method (16). In borosilicate tubes, 250 µL of the extracts’ solution were mixed with 375 µL of AlCl3 (10% in hydroalcoholic solution), 375 µL of 0.1 M Sodium Acetate, and 2,000 µL of ethanol (96%). The mix was allowed to react in the dark for 40 minutes (determined by reaction kinetics). After the incubation, the absorbance at 424 nm was measured in a Thermo Scientific Evolution 60S Spectrophotometer using the previous mixture as a blank but replacing the 250 µL of the sample with ethanol (96%). Each of the determinations was carried out in triplicate.
Statistical analysis
The results were expressed in % radical scavenging activity. The dose-response curves were constructed applying a non-linear regression, and inhibitory concentrations 50 (IC50) in ppm were calculated. The values of the obtained tests were expressed as the mean ± SEM. Data were analyzed using one-way ANOVA and Tukey’s post-hoc. Significant differences were considered for p <0.05. The statistical program GraphPad Prism version 9.0 for Windows (GraphPad Software, San Diego, California USA, www.graphpad.com) was used.
RESULTS
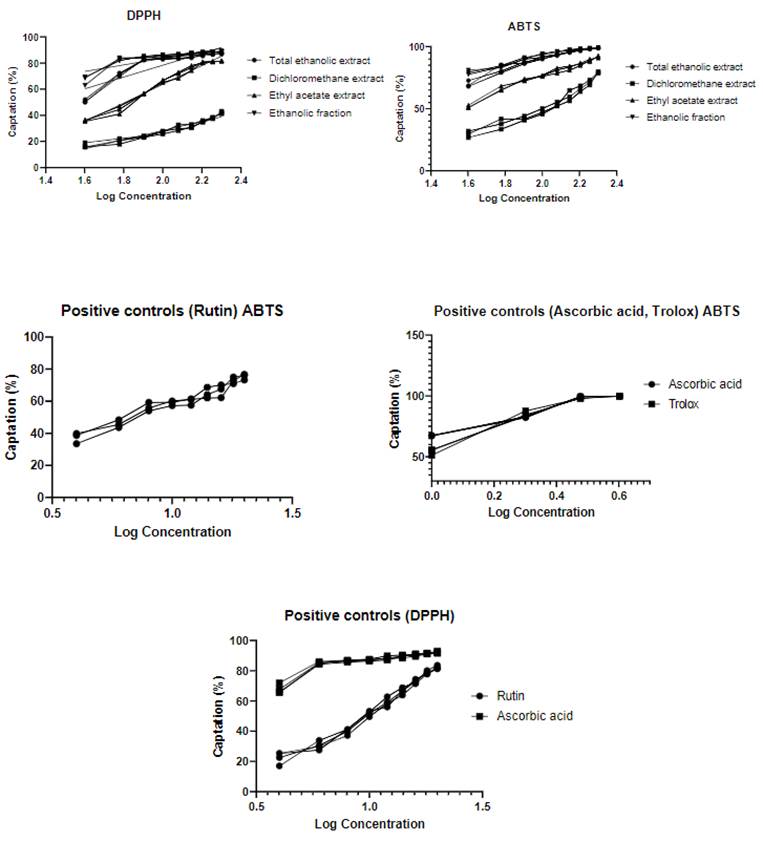
The antioxidant capacity was obtained by capturing the free radicals DPPH• and ABTS•+ using calibration curves of Ascorbic Acid, Trolox, and Rutin (4 to 20 ppm). Of the four extracts, the ethanolic extract (EE) presented inhibition percentages greater than 80% by the DPPH• and ABTS•+ methods, respectively (Table 1). Considering that each method has different reaction conditions, it was possible to infer that the extracts contained various compounds with antioxidant capacity. The obtained results showed that, for all the extracts, the values obtained by ABTS•+ were greater than DPPH•. This could be explained by the ABTS•+ low selectivity since it reacts with any hydroxylated aromatic compound, regardless of its real antioxidant potential. It is important to note that DPPH• becomes more selective than ABTS •+, and unlike the latter, it does not react with flavonoids that do not have hydroxyl groups on the B ring or with aromatic acids that have only one hydroxyl group (17). This could explain the lower values in the DPPH• method compared to the ABTS•+ method.
Table 1 Antioxidant capacity by DPPH• and ABTS•+ (% radical scavenging activity).
| DPPH• assay | ||||
|---|---|---|---|---|
| Concentration (ppm) | Dichloromethane extract (ED) | Ethyl acetate extract (EA) | Total ethanolic extract (TEE) | Ethanolic extract (EE) |
| 40.00 | 16.92 ± 0.08 | 36.02±0.16 | 50.86 ±0.16 | 67.05 ±0.22 |
| 60.00 | 20.30 ± 0.09 | 44.18 ±0.16 | 71.28 ±0.22 | 83.10 ±0.27 |
| 80.00 | 23.58 ± 0.10 | 56.58 ±0.19 | 82.67 ±0.26 | 84.60 ±0.29 |
| 100.00 | 27.03 ± 0.11 | 66.18 ±0.22 | 83.54 ±0.27 | 85.84 ±0.29 |
| 120.00 | 30.3 1± 0.12 | 71.09 ±0.23 | 84.16 ±0.27 | 86.45 ±0.29 |
| 140.00 | 31.53 ± 0.12 | 76.34 ±0.25 | 84.94 ±0.27 | 87.45 ±0.30 |
| 160.00 | 35.35 ±0.13 | 80.54 ±0.27 | 86.17 ±0.27 | 88.03 ±0.30 |
| 180.00 | 38.05 ±0.14 | 81.10 ±0.27 | 86.87 ±0.28 | 88.53 ±0.30 |
| 200.00 | 42.08 ±0.17 | 81.63 ±0.27 | 87.53 ±0.28 | 88.91 ±0.30 |
| ABTS•+ assay | ||||
| 40.00 | 29.43 ±0.10 | 51.50 ±0.16 | 69.77 ±0.18 | 79.27 ±0.22 |
| 60.00 | 37.65 ±0.14 | 66.09 ±0.21 | 81.60 ±0.22 | 83.71 ±0.23 |
| 80.00 | 42.16 ±0.14 | 73.21 ±0.23 | 86.92 ±0.24 | 90.38 ±0.25 |
| 100.00 | 47.63 ±0.16 | 76.46 ±0.24 | 90.74 ±0.26 | 93.14 ±0.25 |
| 120.00 | 53.29 ±0.18 | 80.83 ±0.26 | 93.40 ±0.27 | 95.14 ±0.26 |
| 140.00 | 60.21 ±0.20 | 82.99 ±0.27 | 95.57 ±0.28 | 96.55 ±0.26 |
| 160.00 | 66.13 ±0.22 | 85.87 ±0.28 | 97.03 ±0.28 | 97.85 ±0.27 |
| 180.00 | 71.22 ±0.23 | 88.91 ±0.29 | 98.23 ±0.29 | 98.41 ±0.27 |
| 200.00 | 79.28 ±0.26 | 91.84 ±0.30 | 98.85 ±0.29 | 99.11 ±0.27 |
Table 2 shows the IC50 values expressed in ppm. It was observed that the ethanolic extract (EE) showed lower IC50 values (4.58 and 3.82 ppm, higher antioxidant capacity) for the DPPH• and ABTS •+ method, respectively.
Table 2 IC50 calculated values by DPPH• and ABTS•+
| DPPH• assay | ||||
|---|---|---|---|---|
| Extract and patterns | Electron captation index 50 DPPH• IC50 | Antioxidant activity related to Ascorbic acid AARa Ascorbic Acid | Antioxidant activity related to Rutin AARa Rutin | Antioxidant activity related to Trolox AARa Trolox |
| ED | 414.96 | 789.99 | 45.10 | - |
| EA | 64.18 | 122.20 | 7.00 | - |
| EE | 4.58 | 8.70 | 0.50 | - |
| TEE | 23.58 | 44.90 | 2.60 | - |
| Ascorbic acid | 0.52 | 1.00 | 0.10 | - |
| Rutin | 9.20 | 17.50 | 1.00 | - |
| Trolox | - | - | - | - |
| ABTS•+ assay | ||||
| Extract and patterns | Electron captation index 50 ABTS•+ IC50 | Antioxidant activity related to Ascorbic Acid AARa Ascorbic Acid | Antioxidant activity related to Rutin AARa Rutin | Antioxidant activity related to Trolox AARa Trolox |
| ED | 93.35 | 183.90 | 13.50 | 114.50 |
| EA | 32.89 | 64.80 | 4.70 | 40.30 |
| EE | 3.82 | 7.50 | 0.60 | 4.70 |
| TEE | 10.66 | 21.00 | 1.50 | 13.10 |
| Ascorbic acid | 0.50 | 1.00 | 0.10 | 0.60 |
| Rutin | 6.93 | 0.10 | 1.00 | 8.50 |
| Trolox | 0.81 | 1.60 | 0.10 | 1.00 |
a AAR: relative antioxidant activity using positive controls
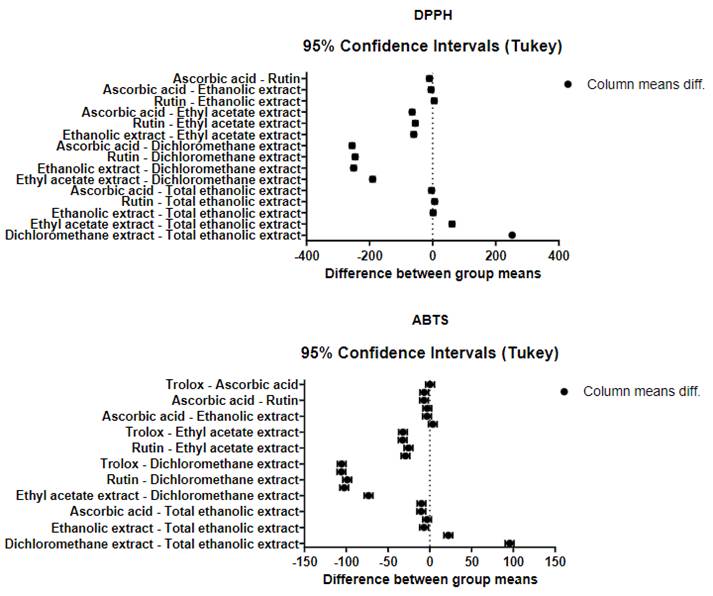
The ethanolic extracts (high polarity) did not show significant differences with respect to the positive controls (Rutin and Ascorbic Acid), by the DPPH• method, according to the statistical analysis (ANOVA, posthoc Tukey; mean difference: TEE vs. Rutin = 6.6 (p = 0.1039); TEE vs. Ascorbic acid = 3.3 (p = 0.7282); EE vs. Rutin = 4.94 (p = 0.3342); SE vs. Ascorbic Acid = 4.96 (p = 0.3295)). A similar behavior was found by the ABTS•+ method (mean difference:
TEE vs. Rutin = 3.2 (p = 0.4084); TEE vs. Ascorbic Acid = 10.19 (p <0.0001, significant); EE vs. Rutin = 3.55 (p = 0.3243); EE vs. Ascorbic acid = 3.33 (p = 0.3971), except for TEE compared to ascorbic acid the mean differences with respect to Trolox were significant (p <0.0001). Alternatively, it is important to highlight that the ethanolic extract (EE) showed a lower IC50 value than the Rutin, by the two methods evaluated (Figure 1).
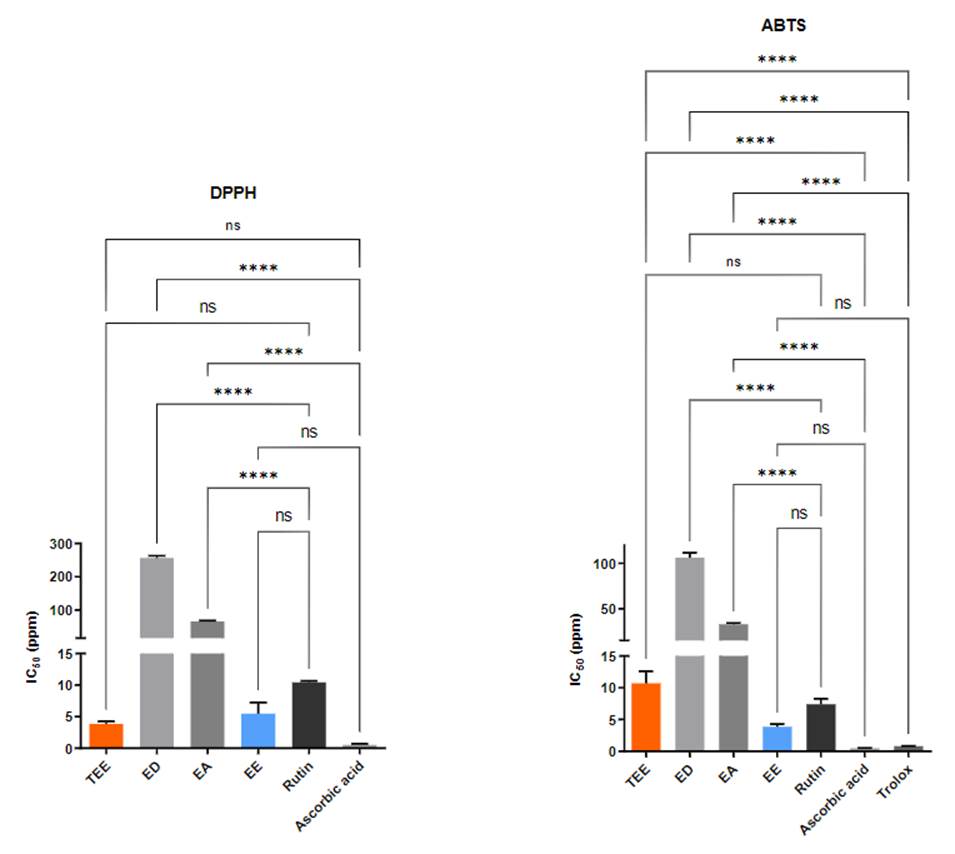
Figure 1 Antioxidant activity - IC50 values for the I. guayusa extracts. Ns-not significant **** p<0,0001(one way ANOVA, post-hoc Tukey).
The values obtained from the quantification of phenols and flavonoids are shown in Table 4. The content of total phenols and flavonoids in plant extracts becomes an important parameter that will establish their antioxidant potential. The Folin- Ciocalteau and AlCl3 complex formation methods permit quantifying the total content of phenolic and flavonoid compounds, respectively, and are used to analyze plant species and foodstuffs (18). The results obtained showed that EA presented the highest levels of phenols, 508.38 ± 1.02 mg Eq of Gallic Acid/g sample, while the flavonoid content for this extract was the lowest of all (16.72 ± 0.32 mg Eq Quercetin/g sample). EE showed a value for phenol content of 357.64 ± 0.60 mg Eq of Gallic Acid/g sample and the highest level of flavonoid content (90.13 ± 5.81 mg Eq Quercetin/g sample).
Table 4 Total phenols and flavonoids content in extracts of I. guayusa.
| Sample | Total Phenols Contenta ± SEM | Total Flavonoids Contentb ± SEM |
|---|---|---|
| TEE | 388.38 ± 0.43 | 30.43 ± 0.87 |
| ED | 198.37 ± 0.15 | 76.58 ± 3.23 |
| EA | 508.38 ± 1.02 | 16.72 ± 0.32 |
| EE | 357.64 ± 0.60 | 90.13 ± 5.81 |
a mg Eq of Gallic Acid/g sample, b mg Eq Quercetin/g sample
DISCUSSION
Many studies have highlighted the antioxidant capacity of polyphenols (7). The antioxidant capacity of I. guayusa leaves was evaluated by electron transfer-based methods such as DPPH• and ABTS•+. Jara et al. (2013) evaluated the antioxidant capacity of guayusa by DPPH• assay to scavenge free radicals and determine the polyphenol composition. They concluded that the antioxidant capacity of I. guayusa ethanolic extracts showed a higher content of phenols and flavonoids, and a higher antioxidant activity (19). These results are comparable with the obtained in this study by the DPPH• method, with the lowest IC50 values reported by the ethanolic extract (EE). Although the ethyl acetate extract presented the highest phenol content, its antioxidant capacity was lower than the ethanolic extract, which may be related to the lower flavonoid content.
The chemistry of phenols has generated great interest in recent years, considering that these compounds are essential, have diverse applications, are antioxidants, and inhibit the oxidative degradation of organic matter, including many aerobic biological organisms (19). Phenolic compounds, being weak acids, act as hydrogen atom donors, capable of reacting with O2• through different mechanisms, depending on their nature and the number of substituents of the phenolic ring, which is why phenols are easily oxidized and act as antioxidants. According to García-Ruíz et al. (2017), I. guayusa reported glycosides flavonols like Quercetin-3- O-hexose, kaempferol-3-O-hexose and quercetin 3-O-rutinoside (7). Likewise, flavonoids present specific characteristics for their free radical scavenging activity, including hydroxyl groups at the C-5 positions and the conjugated double bond at C-2 and C-3 with an oxo function at C-4, and also the two hydroxyl groups of the B-ring (20).
Based on ethnobotanical observations of guayusa, recent analytical and phytochemical investigations have provided information on its nutritional content and chemical composition (21). Phytochemical analyses have identified and quantified secondary metabolites of different types, which have been evaluated using different chromatographic methodologies (HPLC-PDA-MS). García-Ruiz et al. 2017 reported concentrations of carotenoids and phenolic compounds for green leaves (7). Villacís-Chiriboga et al. (2018) compare metabolites between young (2 months) and mature (6 months) leaves (8). Both studies identified and quantified phenolic compounds within hydroxycinnamic acids and flavonols such as quercetin-3-O-hexose and rutin. These results suggest that the high antioxidant capacity observed in ethanolic extracts of guayusa (EE) could be attributed mainly to the presence of polyphenols.
CONCLUSIONS
According to the results obtained in this study, it was possible to corroborate that the ethanolic extract (EE) obtained from the I. guayusa leaves showed the highest antioxidant capacity by the DPPH• and ABTS•+ methods. Additionally, it was possible to relate this capacity to the high flavonoids content present in this extract. Based on the above and future studies, the Ilex guayusa species could be proposed as an important source of antioxidant compounds with possible application in medicine and the food industry.