INTRODUCTION
According to the National Cancer Institute, cancer is one of the leading causes of deaths worldwide (1). The incidence of gastric cancer in Peru is 15.2 per 100,000 inhabitants, being the highest value in all Latin America: Haiti (13.5), Chile (13.0), Colombia (12.8), and Costa Rica (12.8). However, this value is surpassed by Asian countries such as Mongolia (32.5), Japan (31.6), and South Korea (27.9) (2). The 5-year survival of gastric cancer is less than 27%, and it has been estimated that in 2030 it will cause the death of 1.4 million individuals (3).
Annona Muricata (AM) is a medicinal plant belonging to the class Equisetopsida C. Agardhy and subclass Magnoliidae Novák ex Takht (4) and is commonly known as soursop (5). It has been studied for its therapeutic potential and the medicinal properties produced by its secondary metabolites synthesized and located in different parts of the plant (6). Among its various bioactive components, annonaceous acetogenins have anti-proliferative effects and, therefore, potential anti-cancer properties (7,8). These components predominate in the leaves and shell; their extract is being used to search for anticancer properties (9).
The objective of cancer treatment is to inhibit the growth of cancer cells. However, some treatments can damage healthy cells leading to adverse effects that can compromise the patient’s life or do not produce the expected results (10,11). Moreover, these adverse effects can cause greater susceptibility to infections, anemia, neuropathies such as numbness and tingling, decreased mental function, and heart damage (12). To date, many articles have been published on medicinal plants to combat this disease and control its adverse effects (13). According to the World Health Organization (WHO), traditional medicine can reduce costs and be accessible for low-income populations (14).
A preclinical study in mice demonstrated the protective effects of the hydroalcoholic extract of AM on the gastric mucosa reducing the ulceration process by activating prostaglandins and reducing aggressive factors (15). In addition, the cytotoxic effect of the ethanolic extract of AM has been studied in the mouse gastric adenocarcinoma cell line C-678, obtaining favorable results (16). However, no previous study has demonstrated this activity in human gastric adenocarcinoma cells.
Despite the wealth of 5,000 different species of plants that can be used in multiple ways to treat various ailments and consumed as therapy even before approaching a health institution (17,18), there are very few studies demonstrating the real utility of these plants.
Taking all the above into account, the main objective of this study was to determine the cytotoxic effect of AM hydroalcoholic extract (AMOH) and determine the chemical functional groups and the half-maximal inhibition concentration (IC50) against a human gastric adenocarcinoma cell line (AGS).
MATERIALS AND METHODS
We performed an analytical, prospective in-vitro experimental study, as part of a pre-clinical trial design including the AGS cell line.
Preparation of the hydroalcoholic extract from AM
The AM leaves were collected in Alto Amazonas, Bagua and identified at the Centro Nacional de Salud Intercultural of Instituto Nacional de Salud (CENSI/INS). Two hundred grams of leaves were weighed in powder, and 1,500 mL of alcohol 70° were added to the sample and allowed to macerate for 14 days. Then the mix was filtered and reduced to 40° with 20 rpm and 70 mbar in a rotary evaporator RV10C (IKA®) to obtain a resin. The data was collected on an observation sheet (Table 1).
Solubility assay
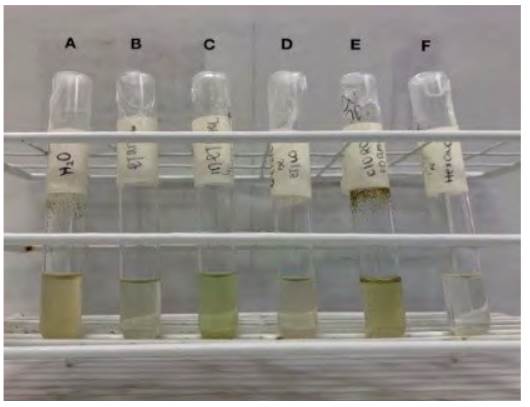
Methanol, ethanol, chloroform, N-hexane, ethyl acetate, and distilled water were added to 50 mg of the dry drug in a proportion of 1 mL to each test tube to determine the solubility of the active components of the sample (19) (Table 2, Figure 1).
Phytochemical screening
Phytochemical screening of the resin of the AMOH was carried out to identify the chemical functional groups (Table 3, Figure 2).
Determination of the total phenolic content in hydroalcoholic extract
Total phenols were determined by the Folin- Ciocalteu method using gallic acid as a reference standard (20). 21.4 mg of the resin was weighed, and a mix of dimethyl sulfoxide (DMSO) and ultrapure water (1:1) was used for resuspension. 100 µL of the sample was taken and diluted in 900 µL of ultrapure water, and the reaction system was performed. The sample was treated three times; 1mL of Folin- Ciocalteau and 0.1 mL of AMOH were added to each sample and set for 5 minutes. Then 1 mL of 7.5% sodium carbonate was added and the sample was placed in a water bath at 45° for 15 minutes. Finally, it was read at 725 nm in a UV/VIS Pharo 300 spectrophotometer (Spectroquant®) (Table 4).
Determination of flavonoids in hydroalcoholic extract
The flavonoids content was determined in AM using quercetin as a reference standard (21). 51mg of the resin was weighed, and a mix of resin and 96% of ethanol was used for the resuspension with a 1:10 solution to perform the reaction system. Three sets of test tubes were prepared and read at 255 nm in a UV/VIS Pharo 300 spectrophotometer (Spectroquant®). A solution of 0.0051 g of quercetin was prepared for the calibration curve in 50 mL of ethanol 96°. The following concentrations were prepared from this stock solution: 1, 5, and 10 ppm (Table 4).
Oncology cell line
The AGS cell line of the European Authenticated Cell Culture Collection (ECACC 89090402) was cultured in Dulbecco´s modified Eagle medium supplemented with 10% of fetal bovine serum and 1% antifungal antibiotic on a stove with CO2/95% and 5% of moisture at 37°C for 24 hours (Figure 3).
Cytotoxic effect
The AMOH dilutions were calculated from a laboratory stock concentration of 32 mg/mL dimethyl sulfoxide (DMSO) stored at -80°C. The human gastric adenocarcinoma cells were counted in the Neubauer chamber (5x103 cells) and placed in a 96-well plate. All the cells were incubated for 12 hours and then 20, 40, 80, and 160 µg/mL of AMOH were added. Morphological changes were microscopically observed and photographed (Nikon Eclipse TI®) after further incubation for 24 and 48 hours. A control group to which 0.5% DMSO was added without the extract was also used. 20 µL of resazurin was added to the wells, and the sample was incubated again for 3 hours to determine cell viability at a concentration of 0.15 mg/mL. Finally, the plate was read by a Synergy LX spectrophotometer (Biotek®) with 570 and 600 nm (Figure 4).
Statistical analysis
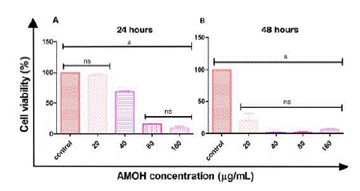
Data obtained from cellular viability absorbance were extrapolated to Microsoft Excel and expressed in percentages considering the control group. Regarding the cytotoxic effect, the response to the different doses and the IC50 were determined by non-linear regression analysis. Significant differences among values were compared with the one-way ANOVA test with post hoc of Tukey test (p<0.05), using GraphPad Prism 5 Project software (Figure 5).
RESULTS
Table 1 Observation sheet of hydroalcoholic extract of Annona Muricata
| Hydroalcoholic extract of Annona Muricata | |
|---|---|
| Initial amount | 1500 mL of 70° alcohol and 200 g of AM |
| Final amount | 32 gr of resin |
| Yield percentage | 16% |
Table 2 Results of solubility assay of the resin of Annona Muricata
| Solvent | Results |
|---|---|
| Methanol | ++++ |
| Chloroform | +++ |
| Ethyl acetate | - |
| N hexane | - |
| Ethanol 70% | ++ |
| Distilled water | ++ |
Legend: ++++: very soluble, +++: soluble in medium proportion, +: soluble in low proportion, -: absent
The results of the phytochemical screening are shown in Table 3 and Figure 2 according to the group of metabolites identified.
Table 3 Phytochemical screening of fresh leaves of Annona Muricata.
| Metabolite | Reactive | Indicator | Results |
|---|---|---|---|
| Free amino acids | Ninhydrin | Coloration | (- -) |
| Carbohydrates (reducing sugar) | Benedict | Coloration | (++) |
| Fehling | Coloration | (++) | |
| Alkaloids | Dragendorff | Coloration | (++) |
| Wagner | Precipitation | (++) | |
| Phenols and Tannins | Ferric trichloride | Coloration | (++) |
| Triterpenes and steroids | Liberman-Buchard | Coloration | (++) |
| Saponins | Foam test | Foam | (++) |
| Quinones | Borntrager | Coloration | (- -) |
| Flavonoids | Shinoda | Coloration | (++) |
| Catechins | Sodium carbonate | Coloration | (- -) |
| Proteins | Biuret | Coloration | (++) |
| Tannins | Gelatin | Precipitation | (++) |
| Cardiotonic Glycosides | Kedde | Coloration | (++) |
| Anthocyanin | Anthocyanin | Coloration | (++) |
| Resin | H20 | Precipitation | (- -) |
(++) Positive, (- -) negative
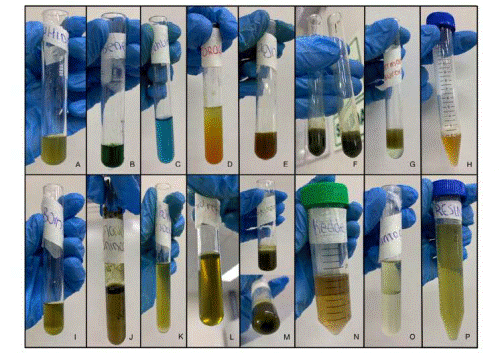
Figure 2 Phytochemical screening. A: Ninhydrin, B: Benedict, C: Fehling, D: Dragendorff, E: Wagner, F: Ferric trichloride G: Liberman- Buchard, H: Foam test, I: Borntrager, J: Shinoda, K: Sodium carbonate, L: Biuret, M: Gelatin, N: Kedde, O: Anthocyanin, P: Resin.
The phenolic compound content in AMOH is shown in Table 4, expressed as mg of gallic acid.
Table 4 Total phenolic and flavonoid content
| Flavonoid content (mg/g of flavonoids sample equivalent to quercetin) | Phenolic content (mg/g of po- lyphenols sample equivalent to gallic acid) |
|---|---|
| 0.249 mg/g | 1.013 mg/g |
Cell morphology
Changes were observed in both cell morphologies. At 24 hours, at a 20 µg/mL concentration, some cells were suspended while others adhered at 24 hours, becoming more notable with the cells being amorphous and fragmented at higher concentrations of 40, 80, and 160 µg/mL. At 48 hours, the effect was much greater, with fragmented cells being observed at the minimum concentration (20 µg/mL) (Figure 3).

Figure 3 Effect of the hydroalcoholic extract of Annona muricata on the morphology of the AGS cell line at 20, 40, 80 and 160 µg/ mL observed at 100X. The control group of cells without extract but with the vehicle (DMSO 0.5%) is shown. Yellow arrow: suspended cells; Green arrow: adhered cells; and White arrow: dead cells.
Cytotoxic effect
The AGS cell line showed cytotoxic activity to AMOH with an IC50 at 24 hours of 45.81 µg/mL and 19.05 µg/ mL at 48 hours (Figure 4). No significant difference was obtained between the cellular control and the response to 20 µg/mL of the extract, with the same being observed for 80 µg/mL versus 160 µg/mL. However, significant differences were observed with the remaining concentrations at 24 hours. At 48 hours, a significant value was obtained between the cellular control and the different concentrations: 20, 40, 80, and 160 µg/mL, with no significant difference among the remaining concentrations (Figure 5).
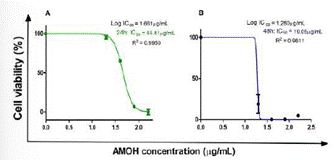
Figure 4 Cytotoxic effect of the hydroalcoholic extract of Annona Muricata (AMOH) on cell viability. The AGS cell line was treated at different concentrations of AMOH (20, 40, 80, and 160 µg/mL) for 24 and 48 hours. The IC50 graphs are shown with a log10 dependent on the cell viability curve of the AMOH concentration (µg/mL).
AMOH: Hydroalcoholic extract of Annona Muricata, IC50: 50% cell inhibition concentration, R2: Coefficient of determination of the non-linear regression model.
DISCUSSION
One of the most important and interesting aspects of our study was determining the chemical and functional groups of AM in the cytoplasm of the plant cell.
The Shinoda reaction was used to identify flavonoids showing a positive faint yellow coloration. Flavonoids have antioxidant activity thanks to a combination of their properties that sequester free iron and chelators, protecting against inflammation. In addition, the flavonoid compounds protect against the formation of malignant tumors, reduce the risk of developing heart disease, and have an antiviral and antibacterial effect (22-24). Alkaloids were identified using the Dragendorff reaction showing a brownish- orange precipitate. The pharmacological spectrum of alkaloids was wide, and the pharmacological properties of these compounds include modification of the nervous system and analgesic, anticancer and antimicrobial activities (25-27). The presence of tannins and phenols was confirmed using the reaction of ferric trichloride, presenting a grayish- green color, identifying the presence of adjacent 2-OH. The defining characteristic of tannins is their astringent capacity, and they are commonly used to treat flu symptoms and bronchitis. They also have pharmacological applications as antimicrobials, antifungals, and antidiarrheals and are used as an antidote for heavy metals. On the other hand, phenols have a long list of properties, the main properties being: antioxidant, anti-allergic, anti- tumoral, and antiasthma, among others (28). The use of the Liebermann-Burchard test allowed identification of triterpenes and steroids, showing an instantaneous change in coloration. These compounds have anti-inflammatory, anti-tumoral, and antiviral activities (29). The presence of peptides and/or proteins was identified by a purple-violet coloration using the Biuret test. These compounds are usually used in patients with stomach disorders, such as infants with malabsorption malnutrition syndrome (30). The use of the foam test identified the presence of both steroid and tripterpenic saponins, for which expectorant, diuretic, and hemolytic actions have been described (27-31). The Kedde reaction identified cardiotonic glycosides related to cytotoxic activity (32). Anthocyanins, which are strong antioxidants, were the last metabolite identified (33). Finally, negative results were obtained for quinones and resin. The presence and absence of all the compounds studied have been corroborated in 6 previous studies in various countries including Brazil, Peru, Venezuela, and Colombia (34-40).
The absence of free amino acids and catechins, which have several medicinal properties, was of note, despite having been reported in previous studies (41,42). Amino acids have regulatory functions in health, such as maintaining immune, gastric, neuromuscular, and cognitive protective functions (43). In the case of catechins, these are excellent antioxidants, help in weight control, prevent cardiovascular diseases, and have anticancer activity (44).
On the other hand, reducing carbohydrates’ presence is unclear since some studies have identified them but not in others. This can be explained by the extract studied; for example, methanol extracts show greater solubility of chemical compounds than ethanolic extracts. In our study, these carbohydrates are related to their high presence in the plant (45).
In the present study, AMOH showed potent cytotoxic activity against the AGS cell line confirmed by our negative control (DSMO 10%). The IC50 at 24 hours was 45.81 µg/mL and 19.05 µg/mL at 48 hours, showing greater cytotoxic activity in vitro at 48 hours. The anti-tumor activity of AM has been studied in various cell lines, showing its cytotoxic activity against breast, prostate, liver, endometrial, skin, colorectal cancer, and hematological neoplasms (46). However, the cytotoxic potential against AGS was evidenced for the first time in this study. This finding is important considering that currently, the regions with the highest mortality per 100,000 inhabitants in Peru are Huánuco (21,7); Huancavelica (17,7), and Junín (16,8), which have a high poverty index and less access to health services, and thus, easy access to this plant could be beneficial (47). Cytotoxic effects of different treatments have been widely studied in different types of cancer and are due to different mechanisms, such as: stimulation of apoptosis through mitochondrial damage, proteins that stop the cell cycle in the G0, G1, and G2 phases, and an increase in the cleavage of caspase-3, with anti-proliferation effects (46). Further studies on the mechanisms inducing cytotoxic effects in gastric cancer are needed.
Additionally, the anti-tumor activity of AMOH can be explained by the total phenol content identified in the present study. In vitro studies have shown that polyphenols have antioxidant effects and can delay or inhibit the oxidation of other molecules making the initiation or propagation of free radical chain reactions impossible and demonstrating their relationship with the anti-proliferative activity of cancer cells (48). Similarly, flavonoids were identified as part of total phenol content using quercetin as a reference, and together these polyphenols enhance the anticancer effects of AMOH.
CONCLUSIONS
AM has chemical functional groups such as flavonoids, reducing carbohydrates, alkaloids, phenols, tannins, triterpenes, steroids, saponins, proteins, cardiotonic glycosides, and anthocyanins with medicinal properties. The cytotoxic effect of AMOH was demonstrated against the AGS cell line with an IC50 of 45.81 µg/mL at 24 hours, achieving the highest effect of 19.05 µg/mL at 48 hours, demonstrating that the effect was maintained. This effect can be explained by metabolites with anticancer properties, mainly phenols, and flavonoids, identified in the present study. Further studies are needed to determine the mechanisms inducing this effect.