Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Suma Psicológica
versión impresa ISSN 0121-4381
Suma Psicol. v.18 n.1 Bogotá ene./jun. 2011
Interdisciplinary research on autism: Behavioral measures in the development of a mouse model of autism
Medidas comportamentales para un modelo de autismo en ratones
María I. Muñoz Blanco1, Linda J. Parrott Hayes1, Kenneth J. Hunter, Jr2
1Department of Psychology, University of Nevada, Reno, USA.
2University of Nevada Medical School, USA.
Correspondence concerning this article should be addressed to Maria I. Muñoz Blanco.
Department of Psychology /296, University of Nevada, Reno
1664 N. Virginia Street, Reno, Nevada 89557, USA.
E-mail: mmunozblanco@unr.edu.
Received: December 5 2010 Accepted: March 10 2011
Abstract
A great deal of biomedical research has provided experimental evidence of the function of specific neuropeptides in the development of autistic symptomatology. Interdisciplinary research in this area would provide a more comprehensive understanding of autism by integrating the findings and contributions from both behavior analysis and biology. In this preliminary interdisciplinary study, pregnant mice were injected with lipopolysaccharide - LPS (experimental group) on the 17th day of gestation and their litters were compared to the litters of mice that were not injected. Measures of social interaction were taken in two periods, namely when the pups were juveniles and when they were young adults, using an ABAB design consisting on the presence/absence of the mother in the chamber. The social interaction of each pup was assessed by observing the number of approaches it made towards its mother. Average velocity, calculated as distance traveled over time, for each mouse during the session was also collected. It was concluded that further refinement of the measure of social interaction was needed, and that a measure of behavioral development may prove useful in the construction of a mouse model of autism.
Keywords: Autism; Animal model; Behavior Analysis; Social Behavior; Interdisciplinary Research.
Resumen
Un gran número de investigación biomédica ha reportado evidencia empírica respecto a la función de neuropéptidos específicos en el desarrollo de la sintomatología del autismo. Se sugiere que con el fin de obtener una mayor comprensión del autismo es necesario establecer un campo de investigación interdisciplinario en el cual es integren contribuciones tanto del Análisis Comportamental como de la Biología. En este estudio interdisciplinario preliminar, ratonas gestantes fueron inyectadas con lipopolisacárido - LPS (grupo experimental) en el 17avo día de gestación. Las camadas de estas ratonas fueron comparadas con camadas control que no fueron inyectadas. Las mediciones de interacción social se realizaron en dos periodos, juvenil y adulto joven, utilizando un diseño ABAB que consistió en la presencia o ausencia de la madre en la cámara experimental. La interacción social de cada cría se evaluó observando el número de acercamientos hacia la madre. El promedio de velocidad, entendida como distancia recorrida sobre tiempo, de cada ratón también fue calculado para cada sesión. Se concluye que es necesario un mayor refinamiento de la medida de interacción social utilizada, y que la introducción de una medida de desarrollo comportamental sería útil en la construcción de un modelo animal de autismo.
Palabras clave: Autismo; Modelo Animal; Análisis Comportamental; Comportamiento Social; Investigación interdisciplinaria.
The epidemiology of autism spectrum disorders (ASD) has shown a significant increase over the past years, from a prevalence of 5 per 10,000 in the 1960s and 1970s to 72 per 10,000 in the 1990s in developed countries (Newschaffer et al., 2007). This increase in the number of children diagnosed with ASD has led scientists from a number of different disciplines to address the possible causes of this disorder and potential treatments for it.
While behavior analytic therapies have been shown to be especially efficacious in the treatment of the clinical aspects of ASD (i.e. Eikeseth, Smith, Jahr & Eldevik, 2007; Lovaas, Koegel, Simmons, & Long, 1973; New Zealand Guidelines Group, 2008, April), these studies have not addressed the causes of these disorders. Moreover, there is some consensus among behavior scientists that there are no obvious environmental circumstances which can fully account for the behavioral anomalies and deficits observed in autistic children as compared to their normally developed peers.
By contrast, a number of biomedical scientists have suggested that there may be a strong genetic component to ASD (Wu et al., 2005) Support for this conclusion comes from the results of twin studies wherein a concordance that ranges from 36% to 91% in monozygotic twins compared to a concordance of less than 1% for dizygotic twins has been found. In addition, overall heritability of 91 to 93% has been calculated and reported for ASD (Bailey et al., 1995; Gale, Ozonoff & Lainhart, 2003). This evidence has led biomedical scientists to study the etiology of ASD.
Research has shown inflammatory changes in the brains of autistic children (Dammann & Leviton, 1997; Jyonouchi, Sun, & Le, 2001; Vargas, Nascimbene, Krishnan, Zimmerman, & Pardo, 2005; Welch et al., 2005); and it has been suggested that one of the principal targets of inflammatory cytokines in the brain is the hippocampus, the limbic structure responsible for coordination of other brain regions, cognition, and memory consolidation (DeLong, 1992). As such it is possible that a disruption of the central processing functions of the hippocampus may lead to the assemblage of clinical findings in autism.
Several research groups have reported that proinflammatory cytokines like TNF-α and IL-1b, whether originating systemically or in the brain, can impair hippocampal neurogenesis (Golan, Lev, Hallak, Sorokin, & Huleihel, 2005). This impairment is evidenced by decreased outgrowth and branching of hippocampal neurons (Neumann et al., 2002), at the point in fetal development (day 16-17 in the mouse) when the hippocampus is making neuronal connections to other brain regions (Soriano, Del Rio, Martinez, & Super, 1994). Although very few neuropathological studies have been done in humans, there is evidence that individuals diagnosed with autism exhibit histologic changes in the hippocampus (Bailey et al., 1998; Bauman & L., 1994; Kemper & Bauman, 1998). In addition, an elevated TNF-α in cerebral spinal fuid in 10 autistic children with a history of regression has been reported, suggesting a chronic inflammatory state of their brains (Vargas et al., 2005). Further, a microarray study found increased transcript levels in many immune system-related genes in a comparison of gene expression in the superior temporal gyrus of six autistic subjects with matched controls (Garbett et al., 2008). Taken together, these findings provide support the hypothesis of a "permanent, abnormal inflammatory state in the autistic brain" (Patterson, Xu, Smith, & Devarman, 2008). As a consequence further research has been conducted with models of maternal immune activation (MIA).
MIA models have successfully demonstrated changes in the hippocampus of these mice compared to control mice (Meyer, Feldon, & Fatemi, 2009). However, biomedical scientists have recognized that the development of adequate behavioral assays for the detection of the aberrant social responses, restricted interests, or repetitive behavior typical of children with ASD has been challenging (Moy & Nadler, 2008). These authors investigated the exploratory behavior of treated mice in a three-chamber experiment where each chamber contained a different stimulus: social (another mouse), leisure (object) or nothing. Moy & Nadler (2008) concluded that, given the relatively low activity levels and reduced exploration on the parts of the treated mice, their measurements were inadequate for the detection of behavioral deficits typical of persons with ADS. In short, adequate murine models of ADS are in need of more refined measures of symptomatic behavior.
Such measures are the hallmark of the science of behavior. Hence, it is asserted that collaborations among behavior analytic and biomedical researchers may be especially fruitful in this domain. It seems likely that, by virtue of these collaborations, an adequate animal model of ADS may be developed and investigated with the aims of discovering causal factors for this disorder as well as potential intervention strategies (Hayes & Delgado, 2007).
The present study, reflecting a preliminary collaboration between behavior scientists and immunologists, was conducted in pursuit of these aims. More specifically, we sought to determine if the neuropathology of ADS is caused, at least in part, by the brain's response to inflammation. Without treatment, autism is an early onset, life- long, profound disability characterized by impaired social development; delayed and deviant language development, and stereotypical patterns of behavior (Rutter, 1978). For present purposes, impaired social development, defined as approaches to other animals including the mother, was selected as the behavioral indicator of ADS.
Method
Subjects
Three female mice and their 24 pups served as subjects. One mother and her pups served as experimental subjects, the remaining two mothers and their pups served as controls. On the 17th day of her pups' gestation, the experimental mother was injected with 75 micrograms per kilogram (75 mg/kg) of lipopolysaccharide (LPS). She delivered 8 experimental offspring including four males and four females. The control mothers were not manipulated during their pregnancies, providing 16 offspring of eight females and four males as control subjects.
The pups were housed with their mothers until weaned, after which the mothers were removed and housed in individual chambers. Additionally, once weaned, the pups were housed with their litter mates of the same gender. The housing room was maintained at 23°C on a 12 hour light/dark cycle. Free food and water were available in the home cages. All procedures were conducted in strict compliance with the policies on animal welfare of the National Institutes of Health.
Social observation procedure
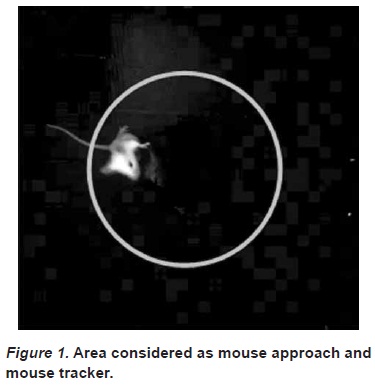
Three days after weaning the social observations began. These observations were conducted in a lit experimental chamber with removable floors with a plastic container fixed in the middle (See figure 1). A webcam with a resolution of 640x480 megapixels was fixed on top of the chamber to track the position of the mouse.
Social observations of individual experimental and control pups were conducted for 10 minute periods at 8:00 am and 6:00 pm over four successive days in an ABAB pattern. During baseline sessions (A), individual subjects were placed in the experimental chamber alone. During intervention sessions (B), individual subjects were placed in the experimental chamber with the pup's mother held in the plastic container.
Social observations, as described above, were conducted at two times, 3 days post weaning (at 3 weeks of age) referred to as the Juvenile measurement, and at 5 weeks of age, referred to as the Young Adult measurement.
Data analysis
The videos for these sessions were decompiled by using SC Video Decompiler Version 6.0.4.2 and a Visual Basic 2008 program designed specifically for this study. Every tenth frame was saved. These frames were analyzed using a computer Mouse Tracker program which provided the space coordinates of the center of the mouse for each frame. These coordinates were used to calculate the distance between the center of the box and the center of the mouse. The mouse was considered to approach the center if the distance with respect to the center was less than 140 pixels (see figure 2). The percentages of frames in which approaches were observed were calculated by comparing the ratio of "approaches" to "non-approaches." Velocity was calculated by measuring the distance travelled over time as recorded on the video.
Results
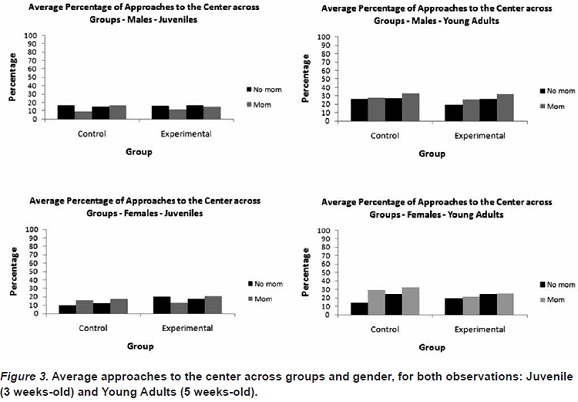
Approaches to the center by males at the Juvenile measurement revealed no differences between the experimental and control subjects, with both groups approaching the center more often when the mother was absent. Differences between the groups were also not found at the Young Adult measurement, although approaches to the center when the mother was present increased for both groups over that observed at the Juvenile measurement.
Approaches to the center by females at the Juvenile measurement were differentiated with the control subjects approaching the center more often when the mother was present than when absent, with the opposite pattern observed for the experimental subjects. The control subjects showed the same pattern of approaches at the Young Adult measurement. This was not the case for the experimental subjects at the Young Adult measurement. Rather, approaches when the mother was present increased, matching those observed in her absence.
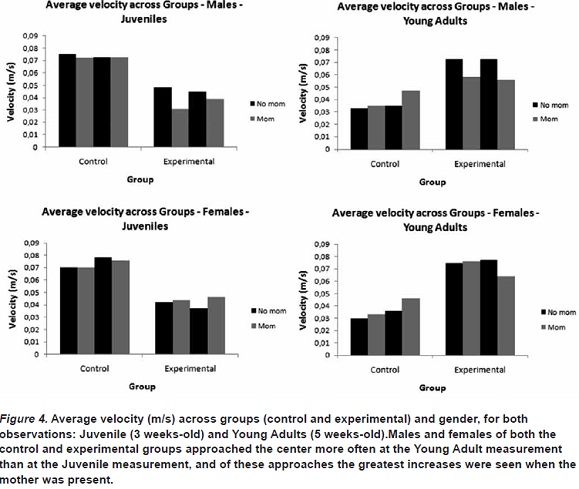
A second measure, velocity, was taken to reflect the relative movement of the two groups. In general, the control group moved much more quickly as Juveniles than as Young Adults, with an average velocity of 0.06 m/s compared to 0.04 m/s, respectively. The opposite pattern was observed for the experimental group. The average velocity for the experimental group as Juveniles was 0.02 m/s compared to 0.06 m/s as Young Adults.
No differences were found in the movements of the control group with respect to the presence and absence of the mother at either measurement. The males in the experimental group showed greater average velocity in the absence of the mother at both measurements. The females in the experimental group showed greater velocity of movement in the presence of the mother than in her absence at the Juvenile measurement. This pattern was not observed in the Young Adult measurement, where no differences were observed.
Anecdotally, the experimental subjects were much more difficult to capture and handle, and much more likely to bite than the control subjects.
Discussion
The present study was conducted to determine if differences in social behavior would be observed between treated and untreated subjects, with the aim of developing a mouse model of autism. Social interaction was defined as approaches to the mother. It was hypothesized that these would be lesser for the treated than for the untreated subjects. While this hypothesis was not confirmed by the present study, the presence of the mother did impact the behavior of the subjects in both groups in unexpected ways. More specifically, as juveniles, both control and experimental males approached the center less often when the mother was present. This was also the case for the juvenile experimental females. This pattern was not observed for any of these subjects as Young Adults. However, approaches to the mother increased substantially for the young adult males. It is possible that these increases reflect sexual maturity. Because approaches to the center were not consistently differentiated by the presence and absence of the mother in either the control or the experimental groups, the measure of social interaction employed in the present study needs further refinement to be useful as a reliable indicator of social deficit.
The velocity data revealed interesting differences between the control and experimental subjects. The movement of the experimental subjects as juveniles was considerably less than that of the juvenile controls. Movement deficits of this sort have been observed in other experiments in which the same preparation was used (Moy & Nadler, 2008), suggesting some impact of the treatment of these animals. Further, the pattern of movement observed in the control groups across the two age-related measurements is consistent with ordinary development in which movement tends to decrease with age. The opposite pattern was observed in the experimental subjects. This pattern cannot be explained as a recovery from the effects of the treatment for two reasons. First, the movement of the experimental group at the Young Adult measurement is much greater than that of the control group; and secondly, the experimental males' movements are differentiated in the presence and absence of the mother. Alternatively, the present study suggests that a measure of behavioral development may prove useful in the construction of a serviceable animal model of autism.
The value of constructing animal models of human disabilities and diseases is in the possibilities they may afford for the investigation, prevention and treatment of these conditions. With regard to investigation as to the causes of human conditions, much of what needs to be done to isolate causal factors cannot be done with human participants for ethical reasons. More importantly, these investigations are essential to the prevention and treatment of human conditions. In the present case, for example, if ASD is shown to be a result of maternal immune activation, procedures may be implemented to prevent this activation from occurring. Anti-inflammatory agents may be prescribed to pregnant women during the period in which inflammation is known to have a deleterious impact on hippocampal development, thereby preventing ASD in their children.
Animal models of human disorders such as autism cannot be developed by biomedical scientists alone because autism is recognized by behavioral deficits. As such, collaborations among behavioral and biomedical scientists may bear greater fruit than is likely to be achieved by either of these groups working independently. The study hereby presented is part of a much larger program of research that includes measurements of the cytokines processing by the hypothalamus as well as additional behavioral measures pertaining to learning deficits and stereotypy. The results so far presented here show a promising area of research that will not only enrich the understanding of social behavior and cytokines processing, but also the understanding of autism as a syndrome that includes factors of different sciences.
References
Bailey, A., Le Couteur, A., Gottesman, I., Bolton, P., Simonoff, E., Yuzda, E., et al. (1995). Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine, 25(1), 63-77. [ Links ]
Bailey, A., Luthert, P., Dean, A., Harding, B., Janota, I., Montgomery, M., et al. (1998). A clinicopathological study of autism. Brain, 121 (Pt 5), 889-905. [ Links ]
Bauman, M. L., & L., K. T. (1994). Neuroanatomic observations of the brain in autism. In M. L. Bauman & K. T. L. (Eds.), The Neurobiology of Autism (pp. 119-145). Baltimore: Johns Hopkins University Press. [ Links ]
Dammann, O., & Leviton, A. (1997). Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatric Research, 42(1), 1-8. [ Links ]
DeLong, G. R. (1992). Autism, amnesia, hippocampus, and learning. Neuroscience Biobehavioral Reviews, 16(1), 63-70. [ Links ]
Eikeseth, S., Smith, T., Jahr, E., & Eldevik, S. (2007). Outcome for children with autism who began intensive behavioral treatment between ages 4 and 7: a comparison controlled study. Behavior Modification, 31(3), 264-278. [ Links ]
Gale, S., Ozonoff, S., & Lainhart, J. (2003). Brief report: pitocin induction in autistic and nonautistic individuals. Journal of Autism and Developmental Disorders, 33(2), 205-208. [ Links ]
Garbett, K., Ebert, P. J., Mitchell, A., Lintas, C., Manzi, B., Mirnics, K., et al. (2008). Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiology of Disease, 30(3), 303-311. [ Links ]
Golan, H. M., Lev, V., Hallak, M., Sorokin, Y., & Huleihel, M. (2005). Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology, 48(6), 903-917. [ Links ]
Hayes, L. J., & Delgado, D. (2007). Invited commentary on animal models in psychiatry: animal models of non-conventional human behavior. Behavior Genetics, 37(1), 11-17. [ Links ]
Jyonouchi, H., Sun, S., & Le, H. (2001). Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. Journal of Neuroimmunology, 120(1-2), 170-179. [ Links ]
Kemper, T. L., & Bauman, M. (1998). Neuropathology of infantile autism. Journal of Neuropathology. Experimental Neurology, 57(7), 645-652. [ Links ]
Lovaas, O. I., Koegel, R., Simmons, J. Q., & Long, J. S. (1973). Some generalization and follow-up measures on autistic children in behavior therapy. Journal of Applied Behavior Analysis, 6(1), 131-165. [ Links ]
Meyer, U., Feldon, J., & Fatemi, S. H. (2009). In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neuroscience and Biobehavioral Reviews, 33(7), 1061-1079. [ Links ]
Moy, S. S., & Nadler, J. J. (2008). Advances in behavioral genetics: mouse models of autism. Molecular Psychiatry, 13(1), 4-26. [ Links ]
Neumann, H., Schweigreiter, R., Yamashita, T., Rosenkranz, K., Wekerle, H., & Barde, Y. A. (2002). Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. The Journal of Neuroscience, 22(3), 854-862. [ Links ]
New Zealand Guidelines Group. (2008, April). The effectiveness of applied behaviour analysis interventions for people with autism spectrum disorder. Systematic Review. Wellington: New Zealand Ministry of Education. [ Links ]
Newschaffer, C. J., Croen, L. A., Daniels, J., Giarelli, E., Grether, J. K., Levy, S. E., et al. (2007). The epidemiology of autism spectrum disorders. Annual Review of Public Health, 28, 235-258. [ Links ]
Patterson, P. H., Xu, W., Smith, S. E. P., & Devarman, B. E. (2008). Maternal Immune Activation, Cytokines and Autism. In A. W. Zimmerman (Ed.), Autism - Current Theories and Evidence (pp. 289-308). Totowa, NJ: Humana Press. [ Links ]
Rutter, M. (1978). Diagnosis and definition of childhood autism. Journal of Autism and Child Schizophrenia, 8(2), 139-161. [ Links ]
Soriano, E., Del Rio, J. A., Martinez, A., & Super, H. (1994). Organization of the embryonic and early postnatal murine hippocampus. I. Immunocytochemical characterization of neuronal populations in the subplate and marginal zone. The Journal of Comparative Neurology, 342(4), 571-595. [ Links ]
Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W., & Pardo, C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology, 57(1), 67-81. [ Links ]
Welch, M. G., Welch-Horan, T. B., Anwar, M., Anwar, N., Ludwig, R. J., & Ruggiero, D. A. (2005). Brain effects of chronic IBD in areas abnormal in autism and treatment by single neuropeptides secretin and oxytocin. The Journal of Molecular Neuroscience, 25(3), 259-274. [ Links ]
Wu, S., Jia, M., Ruan, Y., Liu, J., Guo, Y., Shuang, M., et al. (2005). Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry, 58(1), 74-77. [ Links ]