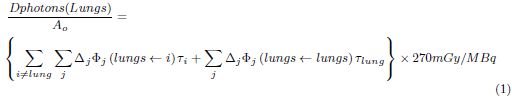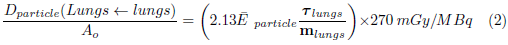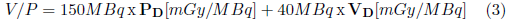Introduction
Nuclear medicine is a mature medical specialty where radioisotopes are used to diagnose and to treat diseases, as well as to carry out scientific research. In order to obtain images a small amount of a pharmaceutical compound with a radioisotope (radiopharmaceutical or radiotracer) is administrated by inhalation, ingestion or injection [1,2].
The radiopharmaceuticals that interact directly with human metabolism are distributed among the organs and tissues of the body according to their biokinetics (biokinetic organs). Radiations emitted by the radiopharmaceuticals interact with organs and tissues of the body, and the absorbed dose depends upon the amount of the administrated radioactive material, the radionuclide decay scheme and its bio kinetics. In order to calculate the absorbed dose in the organs and tissues are used the biokinetic models and the radiopharmaceutical data that are available in the literature [3-8].
So far the internal dose cannot be measured directly, and none operational quantity has been defined to stand in their place. Calculation of the absorbed dose requires mathematical evaluation using mathematical equations and models that simulate human metabolism [9].
There are several methods used for the internal dosimetric calculation; however, the MIRD (Medical Internal Radiation Dose) procedure is mostly used. With MIRD the absorbed dose in an organ is calculated due to radiation received from one or more source organs in the organism [10,11].
To estimate the absorbed dose in adult pulmonary studies 81mKr, 133Xe, 99mTc-Technegas (carbon suspension)-aerosol, and 99mTc- (DTPA)-aerosol radiopharmaceuticals are used to evaluate the pulmonary ventilation (air distribution in the respiratory ducts). To evaluate the distribution of blood flow in the lungs (perfusion) are used macro aggregates (MAA) or 99mTc labeled albumin microspheres (MSA). For diagnosis purpose in adults with suspected pulmonary embolism, the study should combine pulmonary ventilation/perfusion [12,13].
The objective of this work is to determine the procedure that delivers the minimum radiation dose to adult patients with suspected of pulmonary embolism whose study is carried out with radiopharmaceuticals used for Ventilation/Perfusion diagnosis study. The doses were calculated using the MIRD methodology and anthropomorphic representation of Cristy and Eckerman [14].
Materials y Methods
The 99mTc is disintegrated by isomeric transition by gamma emission, β, with an energy of 140 keV and a half-life of 6 hours. The 81mKr decays by isomeric transition emitting 7 with an energy of 190 keV and a half-life of 13 s, gamma radiation can transfer energy directly to one of the most tightly bound electrons, expelling it from the atom, a process named internal conversion. The Xe133 is essentially a β-emitter that decays emitting 7 radiation of 81 keV fundamentally, and a half-life of 5.2 d. [15].
Photons and particles emitted by radioisotopes have a different interaction mechanism with matter; they also have different ranges in the tissues. Therefore, the MIRD procedure was applied using the lungs (target organ) where the absorbed dose per unit of activity was calculated using equation 1.
On the right side of the equation, the absorbed dose represents the dose to the lungs due to the source organ i and its self-dose. In the equation, ∆ j is the average energy of photon j emitted by 99mTc, 133Xe o 81mKr by decay, Φj (lungs ← i) is the fraction of energy of the photon j, emitted by the organ i that is absorbed by the lung per unit mass of the lung; it is also known as the Specific Absorbed Fraction (SAF) [14], and T i is the residence time of the radiopharmaceutical in the source organ i. The absorbed dose, in the lungs due to conversion electrons and Auger electrons was calculated using equation 2.
Here, Ē particle is the average energy of the particle, T lungs residence time of the radiopharmaceuticals in the lungs; while m lungs is the mass of the lungs of an adult.
The most significant residence times for 81mKr, 133Xe, 99mTc (technegas), the 99mTc (MAA) in the biokinetic organs used in dose calculations are shown in Table 1[6,16]. This table includes times of residence for 99mTc (DTPA)-aerosol and 99mTc (MSA) [17].
TABLE 1 Residence time in hours used as organs of biokinetic [6,16,17]
| 99mTc (MAA) | TB (excl. bladder) 7.610 | Lung 4.890 | Liver 1.040 | Kidney 0.018 | Blader content 0.217 |
| 99mTc (Technegas) | Lungs 8.0 | ULI(cont) 0.024 | Stomach cont 0.019 | Remain tissue 0.069 SI content 0.016 | |
| 133Xe (5 minutes) | Lungs 0.013 | Remain tissue 0.533 | |||
| Kr81m (gas) | Lungs 0.00528 | ||||
| 99mTc (DTPA) aerosol | Lung Wahout 1.58 | Bladder content (2.4hs-void) 0.606 | Remainder of body 0.502 | Kidney 0.0394 | |
| 99mTc (MSA) | Lung 4.28 | Stomatch 0.429 | Kidney 0.672 | Bladder 0.202 |
TB: Whole Body: SI: Small intestine; ULI: Upper large intestine.
The dosimetric behavior of radiopharmaceuticals is related to the residence times of the organs of their biokinetic, which in turn, related to differences in biological and physical excretion mechanisms.
In the Tables 2 and 3 are shown the characteristics of photons, and particles emitted in the decay of 99mTc, 133Xe and 81mKr [16] that were used in the dose calculation.
TABLE 2 Nuclear data of emitted photons (MeV) of 99m Tc, 133 Xe and 81m Kr more significant [16] .
| Photons | Ek (MeV) | n k part/dis | ∆k=2.13 n k E k (rad - g) μCi - h | |
|---|---|---|---|---|
| 99mTc | Gamma Radiation | 0.1405 | 0.8906 | 0.2665 |
| 0.1426 | 0.0002 | 0.0001 | ||
| Characteristic radiation | 0.0183 | 0.021 | 0.0008 | |
| 0.0184 | 0.040 | 0.0016 | ||
| 0.0206 | 0.012 | 0.0005 | ||
| 133Xe | Gamma Radiation | 0.1606 | 0.0007 | 0.0002 |
| 0.0796 | 0.0027 | 0.0004 | ||
| 0.0810 | 0.3800 | 0.0656 | ||
| Characteristic radiation | 0.0306 | 0.1410 | 0.0092 | |
| 0.0310 | 0.2620 | 0.0173 | ||
| 0.0350 | 0.0940 | 0.0070 | ||
| 81mKr | Gamma Radiation | 0.1905 | 0.6761 | 0.274 |
| Characteristic radiation | 0.0127 | 0.098 | 0.0027 | |
| 0.0126 | 0.0507 | 0.00136 | ||
| 0.0141 | 0.0150 | 0.00044 |
TABLE 3 Nuclear data emitted particles (MeV) of 99m Tc , 133 Xe and 81m Kr [16] .
| Particles | Ek (MeV) | n k part/dis | n k E k Mev/dis | E particle = Σn k E k Mev/dis | |
|---|---|---|---|---|---|
| 99mTc | Conversion electrons | 0.1195 | 0.0880 | 0.01052 | 0.01446 |
| 0.1216 | 0.0055 | 0.00067 | |||
| 0.1375 | 0.0107 | 0.0015 | |||
| 0.1396 | 0.0017 | 0.00024 | |||
| 0.1400 | 0.0019 | 0.00026 | |||
| 0.1404 | 0.0004 | 0.00006 | |||
| 0.1421 | 0.0003 | 0.00004 | |||
| 0.0016 | 0.7460 | 0.00120 | |||
| Auger electrons | 0.0022 | 0.102 | 0.00022 | 0.00054 | |
| 0.0155 | 0.0207 | 0.00032 | |||
| 133Xe | Beta | 0.0750 | 0.0081 | 0.00061 | 0.1001 |
| 0.1005 | 0.9900 | 0.09949 | |||
| Conversion electrons | 0.0436 | 0.0041 | 0.00018 | 0.03284 | |
| 0.0450 | 0.5510 | 0.02479 | |||
| 0.0753 | 0.0820 | 0.00617 | |||
| 0.0798 | 0.0169 | 0.00135 | |||
| 0.0808 | 0.0044 | 0.00035 | |||
| Auger electrons | 0.0035 | 0.5100 | 0.00178 | 0.00326 | |
| 0.0255 | 0.0582 | 0.00148 | |||
| 81mKr | Conversion electrons | 0.1761 | 0.2690 | 0.0473 | 0.051 |
| 0.1885 | 0.0251 | 0.00475 | |||
| Auger electrons | 0.0106 | 0.0110 | 0.166 x10-4 | 4.54 x10-4 | |
| 0.0107 | 0.0064 | 0.68 x10-4 | |||
| 0.0108 | 0.0342 | 3.7 x10-4 |
The mass of the organs included in adult biokinetics used in the calculations indicates that the mass of the lungs is 1000 g and the mass of the whole body (TB) is 73700 g. [6,14].
For diagnostic purposes in adults with suspected pulmonary embolism, the study should combine pulmonary ventilation/perfusion (V/P) agents [12,13].
In the ventilation/perfusion studies normally are used 150 MBq for perfusion agents and 40 MBq for ventilation agents [18-22].
The absorbed dose due to ventilation/perfusion studies (V/P) was calculated using equation 3:
PD and VD are the doses absorbed by the lungs due to the P (perfusion) and V (ventilation) agents given in mGy/MBq.
Dose results due to V/P agents are given in mGy.
Results
Using the MIRD methodology and the biokinetic characteristics of the pulmonary ventilatory agents 81mKr, 133Xe, 99mTc (tecnegas), 99mTc (DTPA) - aerosol, and perfusion agents 99mTc (MAA) and 99mTc (MSA), the absorbed doses per unit of activity administered to the lungs are determined for each of these agents. Their autodose and the organ dosimetric contributions of their biokinetics are determined.
Table 4 shows the absorbed dose in the lungs, due to photons and radiopharmaceutical particles during perfusion and ventilation studies. Show the absorbed dose, due to photons and particles, in the adult lung during perfusion and ventilation study.
TABLE 4 Absorbed dose per unit of activity administered in the lungs of the adult patient due to perfusion agents 99m Tc(MAA) and 99m Tc (MSA), and ventilation agents 133 Xe, 81m Kr, 99m Tc (Technegas), and 99m Tc (DTPA)-aerosol.
| RFM | emissions | D(lung ← lung)/Ao | D(lung ← i)/Ao | Total mGy/MBq |
|---|---|---|---|---|
| 99mTc (MAA) | Radiation: γ + X | 0.0203(30.2%) | 0.00048 (7.0 %) | 0.067 |
| e-CI + e- Auger | 0.04217(62.9%) | - | ||
| Self-dose | 0.06250 (93.0%) | |||
| 99mTc (MSA) | Radiation: γ + X | 0.0178 (32.4%) | 0.000346 (0.6 %) | 0.055 |
| e- CI + e- Auger | 0.0369 (67 %) | |||
| Self-dose | 0.0547 (99.4 %) | |||
| 99mTc Technegas | Radiation: γ + X | 0.0338 (32.8%) | 0.00007 (0.1 %) | 0.103 |
| e-CI + e- Auger | 0.0690 (67.0%) | - | ||
| Self-dose | 0.1028 (99.8%) | |||
| 99mTc (DTPA) | Radiation: γ + X | 0.0065 (32.2%) | 0.00019 (1 %) | 0.0202 |
| e-CI + e- Auger | 0.0135 (66.8 %) | |||
| Self-dose | 0.020 (99.0%) | - | ||
| 133Xe | Radiation: γ + X | 0.00001 (0.5%) | 0.00012 (11 %) | 0.0011 |
| β+e-CI + e- Auger | 0.00097 (88.3%) | |||
| Self-dose | 0.00098 (89%) | |||
| 8imKr | Radiation: γ + X | 0.00003 (15%) | 0.00020 | |
| e- CI + e- Auger | 0.00017 (85%) | |||
| Self-dose | 0.00020 (100%) |
Table 5 shows the absorbed dose per unit of activity administered in lungs of the adult patient with suspected pulmonary embolism undergoing a perfusion/ventilation study.
TABLE 5 Radiation dose in the lungs during Ventilation/Perfusion patients with suspicion of pulmonary embolism for activities of 150MBq (perfusion), and 40 MBq (ventilation).
| Equation: | *V/P = 150 MBq xPd [mGy/MBq ]+ 40 MBq xVd [ mGy/MBq] | |||||||
| Agents P | 99mTc(MAA) | 99mTc (MSA) | ||||||
| Agents V | 99mTc (DTPA) | 99mTc (Technegas) | 133Xe | 81mKr | 99mTc (DTPA) | 99mTc (Technegas) | 133Xe | 81mKr |
| Absorbed dose (mGy) | 10.85 | 14.17 | 10.09 | 10.06 | 9.05 | 12.37 | 8.29 | 8.26 |
*99mTc (DTPA) / 99mTc (MAA) =150MBq x 0.067 mGy/MBq + 40MBq x 0.020 mGy/MBq = 10.85mGy
Discussion
Table 4 shows that in perfusion studies the lowest dose received by the lungs is due to 99mTc (MSA). In ventilation studies the lower dose is when 81mKr is used.
Table 5 shows that the lowest absorbed dose by the lungs with suspected pulmonary embolism is due to 81mKr / 99mTc (MSA), while the highest absorbed dose is due to 99mTc (Technegas) / 99mTc (MAA).
The dose received by the lungs due to 99mTc (MAA) is 0.067 mGy/MBq, the 93 % of it corresponds to its self-dose (63 % to electrons and 30 % to photons); the rest is due to organs involved in the biokinetic: the liver, kidneys, bladder, and the rest of the tissue the most exposed organ is the liver with 0.0007mGy/MBq. The absorbed dose by the lungs due to 99mTc(MSA) is 0.055 mGy/MBq; the 99.4% corresponds to its self-dose, the rest of the organs involved in biokinetics correspond to the stomach wall as the most exposed organ 0.0002 mGy/MBq.
The absorbed dose by the lungs due to 99mTc (Technegas) is 0.103 mGy/MBq, the 99.8% corresponds to its self-dose (67% to electrons and 32.8 % to photons), the most exposed biokinetic organ is stomach wall with 0.00001 mGy/MBq. Likewise, the absorbed dose in the lungs due to 99mTc(DTPA) is 0.0202 mGy/MBq , the 99.0% corresponds to its self-dose (67% to electrons and 32% to photons), and the most exposed biokinetic organ is the bladder with 0.00002 mGy/MBq . Finally, the absorbed dose in the lungs due to 81mKr is 0.0002 mGy/MBq, it represents the 100 % of its self-dose (85% to electrons and 15% to photons); and for 133Xe (rebreathing for 5 min) the absorbed dose is 0.0011 mGy/MBq, the 89% corresponds to its self-dose (66.4% corresponds to beta, 20% to electronic conversion and 2.2% to Auger electrons).
In all cases the radiations emitted by the radioisotopes Kr81 m, Xe133 and Tc99m (characteristic radiation, gamma, conversion electrons and Auger electrons) conversion electrons are found, in addition to the 133Xe beta which most contribute to the self-dose of the lungs in adults.
The dosimetric contributions of the organs that are part of the biokinetic are greater when using 133Xe with 11%, followed by 99mTc (MAA) with 7 %.
Among the modalities to detect pulmonary embolism, both the V/Q scan and the CTPA (computed tomography pulmonary angiography) expose the patient to ionizing radiation as shown in table 5, in general, carries larger radiation doses than the scan V/Q [23].
Conclusions
Using the MIRD methodology and the Cristy-Eckerman representation for adult lungs, the dose to the lungs was calculated. The dose calculation includes the self-dose in the lungs and the dose due to the source organs involved in the biokinetic of 133Xe, 81mKr, 99mTc (DTPA, MAA, MSA and Technegas).
The highest absorbed dose by the lungs comes from its self-dose due to the charged particles produced during the decay of the 99mTc 81mKr y 133Xe
The lowest absorbed dose by the lungs with suspected pulmonary embolism is due to 81 mKr/99mTc (MSA), and the highest dose is due to 99mTc(Technegas)/99mTc (MAA) calculated for usually recommended activities of 150 MBq for agents' perfusion and 40 MBq for ventilation.