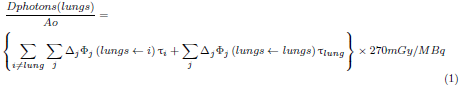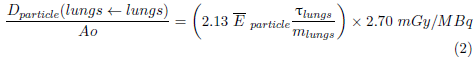Introduction
Radiopharmaceuticals 99mTc (MAA), and 133Xe used in newborn patients with suspected pulmonary embolism are distributed in their organs according to their biokinetics. Dosimetric behavior is related to residence time, which in turn is related to differences in biological and physical excretion mechanisms. The use of radiopharmaceuticals in these patients is exposed to radiation emitted by 99mTc and 133Xe [1-3].
In the MIRD methodology, anatomical models (phantoms) are used to calculate specific absorbed fractions (SAF) using Monte Carlo methods [4]. MIRD phantoms are mathematical representations of the human body. Here, the organs are defined by stylized geometric bodies that describe the sizes and shapes of the organs.
Cristy and Eckermann developed male and female reference phantoms, including newborns, children aged 1, 5, 10, and 15 years, and adults [5]. Phantoms of adult women (nonpregnant and pregnant) were developed by Stabin et al. [6]. The height, body weight, and organ mass of MIRD phantoms are consistent according to the ICRP data [7].
The MIRD phantoms were enhanced by more realistic body models with the use of voxels [8]. Enhanced adult male and female phantoms were developed by Segars [9]. The use of voxels is accompanied by the modified organ masses according to the ICRP data [10]. The Segars model phantoms were based on non-uniform rational b-spline modeling (NURBS) techniques that define a new generation of adult male and female reference models [11,12]. This new set of phantoms was included in the Radiation Dose Assessment (RADAR) resource [13], where the photon SAFs were also included (besides the electrons).
The replacement of MIRD phantoms with updated and improved phantoms raises the question of whether the absorbed dose to the organs also changes. According to Kramer et al. [14], the doses to the organs depend on the geometric similarity of the anatomy of the human body, the elemental composition and density of organs and tissues, and the method of radiation transport used. Therefore, it is necessary to investigate the dosimetric impact of using different phantoms, as well as to evaluate the internal dose administered by available radiopharmaceuticals. Vasquez-Arteaga et al. [15] indicate that "radiopharmaceuticals used during renal studies for a woman with early pregnancy using the Stabin / Segars representations, do not impact the dose absorbed by the kidneys. While the dose absorbed by the uterine wall depends on the representation of Stabin or Segars". Therefore, it is necessary to investigate the dosimetric impact of using different phantoms, as well as to evaluate the internal dose administered by available radiopharmaceuticals.
In neonates, 133Xe is used to assess pulmonary ventilation (distribution of air in the respiratory passages), and 99mTc-labeled albumin macroaggregate (MAA) is used to assess blood flow distribution in the lungs. For diagnostic purposes in newborn patients with suspected pulmonary embolism, the study should combine pulmonary ventilation/perfusion [3,16].
The objective of this work is to determine the absorbed doses in the lungs of newborn patients using 99mTc (MAA) and 133Xe radiopharmaceuticals, the MIRD formalism as well as the Cristy-Eckerman and Segars anatomical representations. The results obtained will be useful to evaluate if there are differences in the use of the SAFs obtained from the anatomical representations of Cristy-Eckerman/Segars.
Materials and methods
The 99mTc disintegrates by isometric transition by gamma emission, with an energy of 140 keV and a half-life of 6 hours. Gamma radiation can transfer energy directly to one of the more tightly bound electrons through internal conversion. The 133Xe is essentially a ß-emitter that decays emitting γ radiation of 81 keV fundamentally, and a half-life of 5.2 d. [17,18].
Photons and charged particles ionize and excite matter with which they interact through different mechanisms that govern the absorbed dose in tissues. In the MIRD procedure, the lungs are assumed as the target organ and the absorbed dose per unit of activity of the administered radiopharmaceutical was calculated using equation 1.
On the right side of the equations, the absorbed dose represents the dose to the lung, due to the source organ i, ∆j is the average energy of the photon j emitted by 99mTc and 133Xe per decay; Φj (lung ←i) is the fraction of energy emitted by organ i that is absorbed by the lung per unit mass of the lung. It is also known as the specific absorbed fraction [19], and Ti is the residence time of the radiopharmaceutical in source organ i.
For charged particles, the absorbed doses to the lungs were calculated using equation 2:
Ē particle is the average energy of the particle, T lungs residence time of the 133Xe and 99mTc (MAA), in the lungs; while m lungs is the mass of the lungs of a newborn.
Target organ and source organ-specific absorbed fractions for C-E and Segars representations in neonates were obtained from Cristy-Eckerman [20], and from RADAR/Stabin et al., [13,21] respectively.
The residence times for 99mTc (MAA) and 133Xe of organs used in the calculations are shown in Table 1[22,23].
Table 1 Residence time in hours used as organs of biokinetic [22,23] .
| 99mTc (MAA) | TB (excl. bladder) | Lung | Liver | Kidney | Bladder content |
| 7.610 | 4.890 | 1.040 | 0.018 | 0.217 | |
| 133Xe | Lungs | Remain tissue | |||
| (5 minutes) | 0.013 | 0.533 |
TB: Whole Body.
Tables 2 and 3 show the characteristics of the photons and particles emitted in the 99mTc and 133Xe decay [24] that were used in the dose calculation.
Table 2 Nuclear data of emitted photons (MeV) of 99m Tc and 133 Xe more significant [24].
| Photons | E k (MeV) | n k part/dis | ∆k = 2.13 n
k
. E
k
|
|
| 99mTc | Gamma Radiation | 0.1405 | 0.8906 | 0.2665 |
| 0.1426 | 0.0002 | 0.0001 | ||
| Characteristic radiation | 0.0183 | 0.021 | 0.0008 | |
| 0.0184 | 0.040 | 0.0016 | ||
| 0.0206 | 0.012 | 0.0005 | ||
| 133Xe | Gamma Radiation | 0.1606 | 0.0007 | 0.0002 |
| 0.0796 | 0.0027 | 0.0004 | ||
| 0.0810 | 0.3800 | 0.0656 | ||
| Characteristic radiation | 0.0306 | 0.1410 | 0.0092 | |
| 0.0310 | 0.2620 | 0.0173 | ||
| 0.0350 | 0.0940 | 0.0070 |
Table 3 Nuclear data emitted particles (MeV) of 99m Tc and 133 Xe [24].
| Particles | E k (MeV) | n k part/dis | n k E k Mev/dis | E particle = ∑ nk E k Mev/dis | |
| 99m Tc | Conversion electrons | 0.1195 | 0.0880 | 0.01052 | 0.01446 |
| 0.1216 | 0.0055 | 0.00067 | |||
| 0.1375 | 0.0107 | 0.0015 | |||
| 0.1396 | 0.0017 | 0.00024 | |||
| 0.1400 | 0.0019 | 0.00026 | |||
| 0.1404 | 0.0004 | 0.00006 | |||
| 0.1421 | 0.0003 | 0.00004 | |||
| 0.0016 | 0.7460 | 0.00120 | |||
| Auger electrons | 0.0022 | 0.102 | 0.00022 | 0.00054 | |
| 0.0155 | 0.0207 | 0.00032 | |||
| 133Xe | Beta | 0.0750 | 0.0081 | 0.00061 | 0.1001 |
| 0.1005 | 0.9900 | 0.09949 | |||
| Conversion electrons | 0.0436 | 0.0041 | 0.00018 | 0.03284 | |
| 0.0450 | 0.5510 | 0.02479 | |||
| 0.0753 | 0.0820 | 0.00617 | |||
| 0.0798 | 0.0169 | 0.00135 | |||
| 0.0808 | 0.0044 | 0.00035 | |||
| Auger electrons | 0.0035 | 0.5100 | 0.00178 | 0.00326 | |
| 0.0255 | 0.058 | 0.00148 |
The mass of the organs included in the newborn biokinetics used in the calculations indicates that the lungs mass of the newborn is 50.6 g, approximate value, according to the ICRP data [10] (does not affect the calculation of particle dose); and the mass of the whole body (TB) is 3600 g [22].
For diagnostic purposes in newborns with suspected pulmonary embolism, the study should combine pulmonary ventilation/perfusion (V/P) agents [25-27].
Results
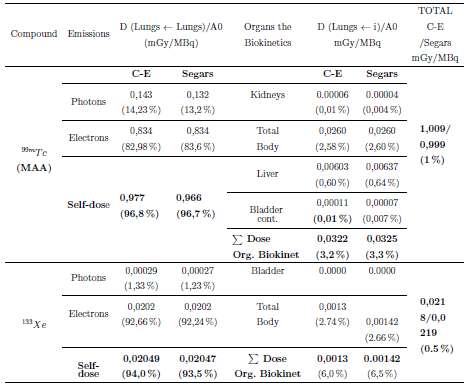
Table 4 shows the absorbed dose in the lungs of newborns, due to photons and particles of radiopharmaceuticals during perfusion and ventilation studies explored with radiopharmaceuticals 133Xe and 99mTc (MAA), for the reference phantoms of Segars and Cristy-Eckerman.
Discussion
The anatomical phantoms of the new generation of Segars clearly represent a significant improvement in the anatomical realism of the organs and it is better modeled in the proximity of the organs, while in stylized models of C-E, the separation of spaces of organs occurs by the simplicity of the forms used to model them [27].
From Table 4, can be noticed that the higher dose absorbed by the lungs in newborn patients explored with 99mTc (MAA) when using the C-E/Segars representations, is mainly due to its autodose with 96.8 % / 96.7 % contribution. The dosimetric contribution due to the organs of biokinetics: kidneys (0.01% / 0.004%), total body (2.58% / 2.60%), liver (0.60% / 0.64%), and bladder (0.01% / 0.007%), are small.
It is observed that the highest dosimetric contribution of the biokinetic organs due to the total body (2.58 % / 2.60 %) is associated with their residence time.
When the lungs are scanned with 99m Tc (MAA), the relative difference in the total dose when using CE and Segars anatomical representations was 1.0%.
The higher dose absorbed by the lungs in newborn patients scanned with 133Xe when using the C-E/Segars representations, is mainly due to its autodose with of 94% / 93.5% contribution. The dosimetric contribution due to its biokinetic organ, the total body (TB), is 6% / 6.5% of the total contribution.
When the lungs were scanned with 133Xe, the relative difference in total dose when using C-E and Segars anatomical representations was 0.5 %.
The highest dose to the lungs for a newborn is due to his self-dose delivered primarily by electrons released by 99mTc and 133Xe.
The use of the C-E or Segars representation is not very sensitively affected by the calculated doses to the lungs of the newborn.
The probable explanation for the behavior of the representations is due to:
The geometric and anatomical differences presented by the CE and Segars representations make the SAF due to the x and 7 photons of 99mTc and 133Xe to be slightly the same, generating insignificant relative differences in the total dose and in the dose of the biokinetic organs.
Conclusions
The absorbed dose in the lungs of newborn patients scanned with radiopharmaceuticals 133Xe (ventilarian) and 99mTc (MAA) (perfusion) is estimated using the MIRD formalism for anatomical representations of Cristy-Eckerman and Segars.
The total dose in the lungs of newborns is due in large part to its self-dose, supplied mainly by the electrons produced during the decay of 99mTc and 133Xe.
For the Cristy-Eckerman and Segars anatomical representations, the relative difference in the total dose between both representations is 1.0 %, when the lungs are explored with 99mTc (MAA).
For the anatomical representations of Cristy-Eckerman and Segars, the relative difference in the total dose between both representations is 0.5 %, when the lungs are explored with 133Xe.
Regardless of the radiopharmaceutical used for pulmonary examinations of a newborn patient, the substitution of the Cristy-Eckerman representation for the Segars representation does not reflect significant changes in the calculation of the absorbed dose in the lungs.