Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Ingeniería
Print version ISSN 0121-4993
rev.ing. no.29 Bogotá Jan./June 2009
Biological Hydrogen Carriers: Harnessing Fossil Fuels in a Carbon-Constrained World1
Bio-combustibles que llevan hidrógeno: Restringiendo combustibles fósiles en un mundo limitado en carbono
Mark T. Holtzapple
PhD. Department of Chemical Engineering, Texas A&M University. m-holtzapple@tamu.edu
Recibido 23 de abril de 2009, modificado 10 de julio 2009, aprobado 20 de junio de 2009.
PALABRAS CLAVES
Alcohol, alcanos, compuestos carboxílicos, conversión, economía, eficiencia energética, MixAlco, procesos con azúcares, procesos termoquímicos.
RESUMEN
Debido a una amplia disponibilidad, el uso de recursos lignocelulósicos como materia prima para producir combustibles y otros productos químicos es muy prometedor. Para hacer de esto una realidad, 18 años de investigación en la Universidad de Texas A&M han resultado en el desarrollo de un proceso novedoso denominado MixAlco.TM Este artículo compara este proceso con otras tecnologías y provee algunos detalles del proceso. Por último, se presenta un breve análisis económico.
KEY WORDS
Alcohol, alkanes, carboxylate platform, economics, efficiency, energy yields, gasification, MixAlco, sugar platform, thermochemical platform.
ABSTRACT
Due to their widespread availability, the use of lignocellulosic resources as feedstocks for fuels and chemicals is very promising. To make this a practical reality, 18 years of research at Texas A&M University have resulted in the development of a novel process known as MixAlco.TM This article compares this process with other technologies and provides some process details. At the end, a brief economic analysis is presented.
INTRODUCTION
Ethanol produced from corn grain and diesel produced from soybean are currently the predominant transportation biofuels in the United States [1]. Globally, the United States (using corn grain) and Brazil (using sugarcane) are the two primary producers of ethanol with 8.1 billion gallons produced in 2005 accounting for about 70% of the world consumption of bio-ethanol [2]. These paths to ethanol production are often criticized because of significant arable land and water requirements, negative environmental impacts, and competition with food resources.
Intensive work has been done to identify feedstock alternative to these resources along with corresponding conversion processes. Lignocellulosic feedstocks are promising because of their potential supply is far larger than that of food crops; consequently, several different technological configurations have arisen to use these feedstocks. In this paper, three alternative processing platforms are considered: thermochemical, sugar, and carboxylate explained as follows:
THERMOCHEMICAL PLATFORM.
Involves partial oxidation to produce synthesis gas (CO + H2), which can be catalytically converted to a variety of products (e.g., alcohols and hydrocarbons). Gasification of ideal biomass may be visualized as occurring according to the following steps: (1) the lignin is separated from the polysaccharide, (2) the polysaccharide is gasified, (3) the lignin is gasified and shifted to hydrogen, and (4) the resulting gases are passed over a catalyst to yield ethanol.
SUGAR PLATFORM.
Employs the enzymatic hydrolysis of cellulose and hemicellulose to sugars, which are subsequently fermented into ethanol. Using the sugar platform to completely convert ideal biomass involves the following steps: (1) carbohydrate polymers are hydrolyzed to sugars using acid or enzyme catalysts, (2) the resulting sugars are fermented to ethanol and carbon dioxide, (3) the remaining lignin is gasified and the resulting gases are shifted to hydrogen, and (4) the hydrogen reduces carbon dioxide to form more ethanol.
CARBOXYLATE PLATFORM.
The cellulose and hemicelluloses are fermented to carboxylic acids, which are subsequently converted into fuels or chemicals. Using the carboxylate platform to completely convert ideal biomass involves the following steps: (1) carbohydrate polymers are hydrolyzed to sugars using enzyme or acid catalysts, (2) the resulting sugars are fermented to acetic acid, (3) the remaining lignin is gasified and the resulting gases are shifted to hydrogen, and (4) the hydrogen reduces the acetic acid to form ethanol.
THEORETICAL YIELDS AND MASS BALANCES
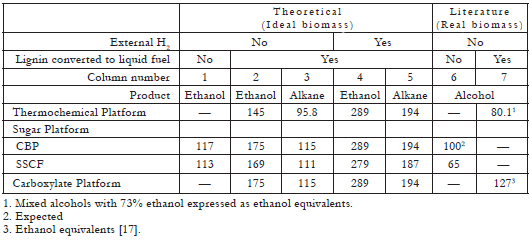
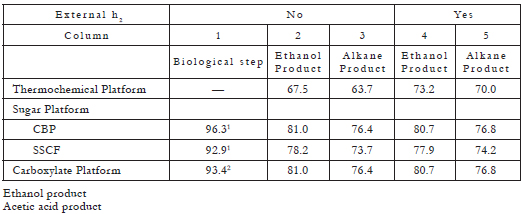
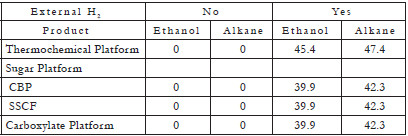
The major components of lignocellulosic biomass are cellulose and other polysaccharides (collectively represented as C6H10O5), and lignin (represented as CH1.12O0.377). Using balanced chemical reactions and the ideal biomass feedstock, which for the purposes of the discussion here, will be defined as 31.7% lignin and 68.3% polysaccharides on an ash-free basis; and consequently a molar ratio of 3.93:1 lignin:polysaccharide (a composition typical of hardwoods, such as poplar wood), the theoretical yield and energy efficiency can be calculated for all three platforms [3]. These results allow for a comparison on the same basis. A summary of such balances is presented in Tables 1, 2 and 3 (For a detailed explanation of these tables, see [3]). The most important results are summarized as follows:
Table 1. Yields of liquid fuels from biomass (gal/ton ash-free biomass) [3].
All of these advantages of the carboxylate platform have been recognized Eggeman and Verser [4], Granda and Holtzapple [5], and Flatt and van Walsum [6], all of whom are pursuing active research projects in this area.
Table 2. Theoretical efficiency of liquid fuels from ideal biomass (%) [3].
Table 3. Theoretical energy supplied from external hydrogen (%) [3].
THE MixAlco PROCESS
The MixAlco process employs a mixed culture of acid-forming microorganisms to convert biomass to carboxylic acids (e.g., acetic, propionic, and butyric acids). Provided a methanogen inhibitor is added, these acids are the low-energy, thermodynamically driven products; therefore, there is no need to maintain aseptic operating conditions. To keep the pH near neutrality, buffers (e.g., calcium carbonate, ammonium bicarbonate) are added; thus, the products of these mixed-acid fermentations are carboxylate salts (e.g., calcium acetate, ammonium acetate). These are concentrated via vapor-compression evaporation and subsequently chemically converted to other chemical and fuel products.
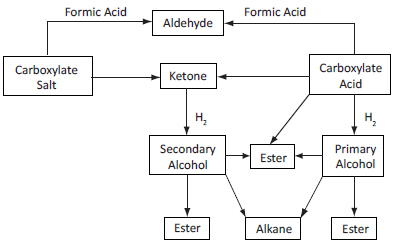
The advantages of the mixed-acid fermentation may be exploited to manufacture chemical and fuel products through the carboxylate platform (Figure 1). Using well-established catalysts and chemistry, the products can be made starting either from the carboxylate salt or the carboxylic acid. Transforming ketones or carboxylic acids into alcohols requires energy input from hydrogen. This can be supplied from many sources, including the gasification of undigested residues exiting the fermentor; thus, all biomass components (including lignin) may be transformed into high-value products.
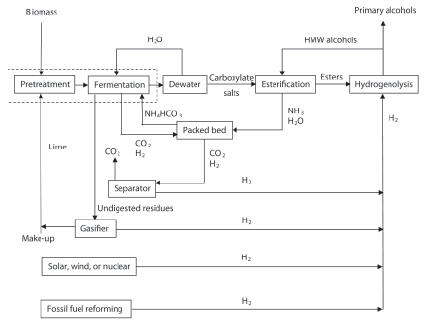
Figure 1. Overview of routes to chemical and fuel products via the carboxylate platform [7].
The process can use a wide variety of feedstocks (e.g., municipal solid waste, sewage sludge, manure, industrial biosludge, food scraps, agricultural residues, energy crops). Other important features of this process are[7]:
PROCESS OVERVIEW
Among the many products of the carboxylate platform are mixed alcohols, which may be used as transportation fuels. Routes to mixed alcohols are called the MixAlco process (Figure 2), which has the following steps (more details of each step can be found elsewhere [7]:
Figure 2. Overview of the MixAlco process [7].
PRETREATMENT
Lignocellulosic biomass is contacted with lime, which removes lignin to enhance reactivity. Low-lignin (<10%) biomass does not require pretreatment. Medium- lignin (10 – 18%) biomass responds well to 1 – 2 h of contact with boiling lime water [8-10]. Highlignin (18 – 24%) biomass responds well to long-term lime plus air (~55oC, 1 month, 1 atm) [11]. Very-highlignin (>24%) biomass requires lime plus oxygen (~150oC, 2 h, ~20 atm) [12, 13]. (Note: In this paper, it is assumed that the feedstock is high-lignin biomass that is pretreated with lime plus air.)
FERMENTATION
Inocula for the mixed-acid fermentation may be derived from soil, particularly from saline environments. The fermentation may be performed in submerged fermentations [14-16] or in piles [17]. Methanogen inhibitors prevent conversion of the carboxylic acids to methane and carbon dioxide. Although calcium carbonate may be used as a buffer, the process shown in Figure 2 uses ammonium bicarbonate. Laboratory experience indicates that high ammonium ion concentrations naturally inhibit methanogens; for safety, iodoform will be added also. Undigested residues from the fermentor may be sent to a gasifier where hydrogen is made. Insoluble calcium carbonate –formed from the reaction of pretreatment calcium and fermentation carbon dioxide– exits with the undigested residues. The ash from the gasifier contains alkaline salts, including calcium oxide (lime), which is used to pretreat the incoming biomass. The fermentor gas contains a mixture of hydrogen (~50 mol%) and carbon dioxide (~50 mol%), which can be easily separated.
DEWATERING
The fermentation broth is concentrated using vapor compression. Operating at elevated temperature and pressure reduces the compressor size.
ESTERIFICATION
In a distillation column, the concentrated ammonium carboxylate salts in the fermentation broth are contacted with a high-molecular-weight (HMW) alcohol and a solid-acid catalyst (e.g., USY-zeolite,). Water and ammonia exit the top of the distillation column allowing esters to form. The ammonia and water are sent to a scrubber where they react with carbon dioxide to form ammonium bicarbonate.
HYDROGENOLYSIS
The esters react with hydrogen using a catalyst (e.g., copper chromite) to form primary alcohols, which are separated in a distillation column. The low-molecular- weight (LMW) alcohols exit the top of the column and are sold as product and the HMW alcohols are recycled to the esterification reactor.
ECONOMICS
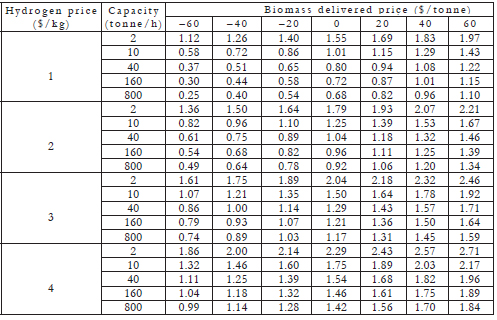
When alcohols are produced by the carboxylate platform, hydrogen is required. It can be supplied from a number of processes, including gasifying biomass, separation from fermentor gases, methane reforming, or electrolysis. Additionally, it can be obtained from renewable sources (solar, wind, nuclear) or reformed fossil fuels (coal, petroleum coke, natural gas). The hydrogen in the fermentor gas is the most economical source, but it supplies only 18% of the needs. To supply the remaining hydrogen needs, the most economical hydrogen production methods operate at large scale (steam reforming of methane, biomass gasification). Unless major breakthroughs occur, electrolysis is the least economical hydrogen source. On the basis of the biomass fed, three scenarios can be considered [7]:
SCENARIO A
Municipal solid waste. The average US tipping fee for disposing of municipal solid waste (MSW) is about $45/dry tonne (assuming 15% moisture) [18]. If it is assumed that the cost of sorting the MSW is offset by the value of recyclables (e.g., aluminum), then the organic fraction of MSW has a net tipping fee of about $45/dry tonne. A base-case plant that processes 40 tonne/h would service a city of about 800,000. If a refinery or hydrogen pipeline is available locally, then hydrogen can be obtained for about $2/kg. According to Table 4, the minimum alcohol selling price would be $0.72/gal. According to Equation 1, the corresponding gasoline selling price would be $1.14/ gal. The capital cost for the alcohol plant is $36.8 million, or $0.82/annual gallon of mixed alcohol.
Table 4. Minimum alcohol selling price (MASP) including 15% return on investment ($/gal) [7].
SCENARIO B
Small energy plantation. A base-case plant of 40 tonne/h could be supplied from 7110 ha (17,600 acre, 27.5 mi2) assuming biomass yields of 45 tonne/ (ha·yr) (20 tons/(acre·yr)), which can be achieved with high-yield sorghum being developed at Texas A&M University. If the land were 50% planted, this would require harvesting within a 7-km (4.2-mi) radius. Assume the delivered biomass cost is $60/tonne and that biomass residue and wood waste ($46/tonne) is gasified to make hydrogen. Further, assume that fermentor gases are a portion of the hydrogen source. Using these assumptions, hydrogen is $2.50/kg. According to Table 4, the minimum alcohol selling price would be $1.59/gal. According to Equation 1, the corresponding gasoline selling price would be $2.41/ gal. The capital cost for the alcohol and gasification plants is $36.8 million and $66.3 million, respectively, or $2.29/annual gallon of mixed alcohol.
SCENARIO C
Large energy plantation processing 800 tonne/h could be supplied from 142,000 ha (351,000 acre, 549 mi2) assuming the same biomass yield (45 tonne/ (ha·yr)). If the land were 50% planted, this would require harvesting within a 31-km (18.6-mi) radius.
Assume the delivered cost is $60/tonne and that biomass residue and wood waste ($46/tonne) is gasified to make hydrogen. Further, assume that fermentor gases are a portion of the hydrogen source. Using these assumptions, hydrogen is $1.42/kg. According to Table 4, the minimum alcohol selling price would be $1.20/gal, and the corresponding gasoline selling price would be $1.85/gal. The capital cost for the alcohol and gasification plants is $496 million and $494 million, respectively, or $1.10/annual gallon of mixed alcohol.
To summarize, using the carboxylate platform within the MixAlco process, a base-case plant processing MSW and using hydrogen from a pipeline or refinery can sell alcohols for $0.72/gal or gasoline for $1.14/ gal; for a small energy plantation, the minimum alcohol selling price would be $1.59/gal, and a large energy plantation can sell alcohols for $1.20/gal or gasoline for $1.85/gal.
CONCLUSIONS
Colombia is extremely rich in lignocellulosic resources. Implementing a process like the MixAlco in Colombia – either in conjunction with a conventional oil refinery or on its own – would have tremendous environmental and economic impacts. The MixAlco process requires no scientific breakthroughs. It is a robust and flexible technology that is commercially ready and is economically competitive, even with oil prices near $60/bbl.
The MixAlco process employs a mixed culture of acid-forming microorganisms to convert biomass to carboxylate salts, which are concentrated via vaporcompression evaporation and subsequently chemically converted to other chemical and fuel products. To make alcohols, hydrogen is required. One of the challenges is obtaining inexpensive hydrogen; however, it can be supplied from a number of processes, including gasifying biomass, separation from fermentor gases, methane reforming, or electrolysis. Using zeolite catalysts, the alcohols can be oligomerized into hydrocarbons, such as gasoline. A 40-tonne/h plant processing municipal solid waste ($45/tonne tipping fee) and using hydrogen from a pipeline or refinery ($2.00/kg H2) can sell alcohols for $0.72/gal or gasoline for $1.14/gal with a 15% return on investment. The capital cost is $0.82/annual gallon of mixed alcohols. An 800-tonne/h plant processing high-yield biomass ($60/tonne) and gasifying fermentation residues and waste biomass to hydrogen ($1.42/kg H2) can sell alcohols for $1.20/gal or gasoline for $1.85/ gal with a 15% return on investment. The capital cost for the alcohol and gasification plants is $1.10/annual gallon of mixed alcohols.
NOTAS AL PIE
1. This article has been written in collaboration with Rocio Sierra, Professor at Universidad de los Andes.
REFERENCES
[1] J. Manuel. "Battle of the biofuels". Environmental Health Perspectives. Vol. 115, No. 2, 2007, pp. 92-94. [ Links ]
[2] B. D. Solomon, J. R. Barnes and K. E. Halvorsen. "Grain and cellulosic ethanol: History, economics, and energy policy". Biomass and Bioenergy. Vol. 31, No. 6, 2007, pp. 416-425. [ Links ]
[3] M. T. Holtzapple and C. B. Granda. "Carboxylate Platform: The MixAlco Process Part 1: Comparison of three biomass conversion platforms". Applied Biochemistry and Biotechnology Vol. 156, No. 1-3, 2009, pp. 95-106. [ Links ]
[4] T.Eggeman and D.Verser. "The importance of utility systems in today's biorefineries and vision for tomorrow". Applied Biochemistry and Biotechnology Vol. 129-132, 2006, pp. 261-381. [ Links ]
[5] C. B. Granda and M. Holtzapple. "Biorefineries for Solvents: The MixAlco process". In Bioenergy, Judy Wall, C. H., Arnold Demain, (Ed). Washington: ASM Press, 2008. [ Links ]
[6] M. Flatt and G. van Walsum. "On-site Demonstration of Lime Pretreatment and Acidogenic Digestion of Dairy Manure for Provision of Carboxylate Salt Platform Chemicals". Manuscript in preparation, 2008. [ Links ]
[7] C. B Granda, M. T. Holtzapple, G. Luce, K. Searcy and D. Mamrosh. "Carboxylate Platform: The MixAlco Process Part 2: Process Economics". Applied Biochemistry and Biotechnology. Vol. 156, No. 1-3, 2009, pp. 107-124. [ Links ]
[8] W. E. Kaar and M. T. Holtzapple. "Using lime pretreatment to facilitate the enzymic hydrolysis of corn stover". Biomass and Bioenergy. Vol. 18, No. 3, 2000, pp. 189-199. [ Links ]
[9] V. Chang, B. Burr and M. Holtzapple. "Lime pretreatment of switchgrass". Applied Biochemistry and Biotechnology. Vol. 63-65, No. 1, 1997, pp. 3-19. [ Links ]
[10] V. Chang, M. Nagwani and M.Holtzapple. "Lime pretreatment of crop residues bagasse and wheat straw". Applied Biochemistry and Biotechnology. Vol. 74, No. 3, 1998, pp. 135-159. [ Links ]
[11] S. Kim and M. T.Holtzapple. "Lime pretreatment and enzymatic hydrolysis of corn stover". Bioresource Technology. Vol. 96, No. 18, 2005, pp. 1994-2006. [ Links ]
[12] V. S. Chang, M. Nagwani, K. Chul-Ho, M. T. Holtzapple. "Oxidative Lime Pretreatment of High-Lignin Biomass". Applied Biochemstry and Biotechnology. Vol. 94, 2001, pp. 1-28. [ Links ]
[13] R. Sierra, C. B. Granda and M. Holtzapple. "Short-Term Lime Pretreatment of Poplar Wood". Biotechnology Progress. Vol. 25, No. 2, 2009, pp. 323-332. [ Links ]
[14] C. Aiello-Mazzarri, F. K. Agbogbo and M. T.Holtzapple. "Conversion of municipal solid waste to carboxylic acids using a mixed culture of mesophilic microorganisms". Bioresource Technology. Vol. 97, No. 1, 2006, pp. 47-56. [ Links ]
[15] S. B. Domke. Fermentation of industrial biosludge, paper fines, bagasse, and chicken manure to carboxylate salts. Thesis (PhD.), Texas A&M University, 1999. [ Links ]
[16] P. Thanakoses, N. Mostafa and M. Holtzapple. "Conversion of sugarcane bagasse to carboxylic acids using a mixed culture of mesophilic microorganisms". Applied Biochemistry and Biotechnology. Vol. 107, No. 1, 2003, pp. 523-546. [ Links ]
[17] F. K. Agbogbo. Anaerobic fermentation of rice straw and chicken manure to carboxylic acids. In Texas A&M University: College Station, Tex., 2007. [ Links ]
[18] E. W. Repa. Tipping Fee Survey, NSWMA Research Bulletin 02-03. Washington, D.C., 2002. [ Links ]