Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Ingeniería
versión impresa ISSN 0121-4993
rev.ing. no.39 Bogotá jun./dic. 2013
Laccase Alginate Encapsulation: Comparison between Assisted and Non-assisted Extrusión for Large-scale Production
Encapsulación de lacasa en alginato: comparación entre extrusión asistida y no asistida para producción a gran escala
Juan C. González(1)*, Adriana P. Ascencio(2)*, Camila A. González-Williamson(3)*, Johann F. Osma(4)*
(1)* Ingeniero Químico. jc.gonzalezl41@uniandes.edu.co
(2)* Ingeniera Electrónica y Eléctrica. ap.ascencio266@uniandes.edu.co
(3)* Ingeniera Electrónica y Eléctrica. ca.gonzalez961@uniandes.edu.co
(4)* Ph.D en Ingeniería Química Ambiental y de Procesos. Profesor Asistente. jf.osma43@uniandes.edu.co
* Universidad de los Andes, Bogotá Colombia.
Received October 19th, 2012. Modified February 2nd, 2013. Approved February 14th, 2013.
Key words
Encapsulation, laccase, extrusion.
Abstract
The effect of assisted and non-assisted extrusión for large-scale production on size, shape and biochemical performance of laccase-alginate beads was studied. Results showed that the extruding technique affeets bead size and size distribution but not the shape of the beads. Biochemical characterization showed a similar performance for both extruding methods. However, non-assisted beads presented higher enzymatic activity variability and lower stability in time. Results showed that using the extrusión method, bio-processes are improved by highly homogeneous particulate material facilitating temperature or pH controlled steps. Thus, an assisted method presents several benefits for producing lacease particulate material in large quantities.
Palabras claves
Encapsulación, lacasa, extrusión.
Resumen
Se estudió el efecto de la extrusión asistida y no-asistida sobre el tamaño, forma y desempeño bioquímico para la producción a gran escala de capsulas de lacasa-alginato. Los resultados mostraron que la técnica de extrusión afecta el tamaño y distribución de las capsulas, pero no su forma. Las capsulas no-asistidas presentaron mayor variabilidad en su actividad enzimática y menor estabilidad en el tiempo. Los resultados demostraron que material particulado altamente homogéneo facilita el control de etapas de temperatura y pH, por lo tanto, un método asistido presenta ventajas para la producción de capsulas de lacasa en grandes cantidades.
INTRODUCTION
Lacease (p-diphenol: oxygen oxidoreductases) is found in fungi, higher plants, inseets and bacteria. Due to their specificity and higher activity to a wide variety of substrates, lacease has the potential to be used in applications such as bioremediation, pulp and paper industry, dye degradation and food monitoring, among others. Nevertheless, free lacease exhibits sensitivity to denaturing agents and environmental disturbances. In addition, the need for large quantities of the enzyme leads to a higher operating variable cost associated with its production and purification described by Osma, Toca-Herrera & Rodriguez-Couto (2011) due to the difficulty in separating it from the residual reaction. These drawbacks limit further industrial application of lacease.
Enzyme immobilization provides a possibility to enhance the operational stability of the biocatalyst, improve the op-erational control and achieve an easier product recovery and flexibility of the reactor design; henee, decreasing operating cost and quantities of the enzyme required (Mohidem & Bin Mat, 2012).
Diverse methods have been employed for lacease immobilization such as entrapment within polymeric gels, absorp-tion on solid supports, covalent bond on a solid substrate and enzyme cross-linking by multifunctional reagents (Fernández-Fernández, Sanromán & Moldes, 2012). Entrapment in alginate beads is one of the most inexpensive, fast and simplest methods to immobilize lacease (Chai, Mei, Lin & Yao, 2004).
The traditional and most extensively used method for entrapment in alginate beads is extrude-dropping (Shilpa, Agrawal & Ray, 2003).
This procedure is carried out by mixing lacease with a so-lution containing alginate acid salt. Then, the mixture is ex-truded through a needle and allowed to drop under gravity into a divalent catión solution. Lacease entrapped in alginate beads has shown to be able to enhance thermal stability, al-low catalyst reutilization, facilítate product recovery, and as-sists in controlling the chemical reaction (Niladevi & Prema 2008; Lu, Zhao & Wang 2007).
Lacease immobilization on alginate beads has been report-ed in dye decolonization by Faraco, Pezzella, Miele, Giardi-na & Sannia (2009), polycyclic aromatic hydrocarbons deg-radation (Dominguez, Gómez, Lorenzo & Sanroma, 2007) and phenol removal (Niladevi & Prema 2008). Commonly, lacease activity and its thermal stability have been compared in these studies without discriminating the method for the alginate bead production.
Some methodological studies focusing on enzyme entrapment by alginate beads reported effeets of the needle nozzle size on alginate bead formation (Radha, Regupathi, Arunag-iri & Murugesan, 2005), divalent catión and the effect of its concentration on the activity immobilization yield (Niladevi & Prema, 2008) , as well as shape and size analysis of the alginate beads (Chan, Lee, Ravindra & Poncelet, 2009). How-ever, there are no studies reported on the distinction between assisted and non-assisted methods for large-scale production of alginate beads by extrude-dripping.
Thus, the aim of this study is to analyze differences between assisted and non-assisted extrude-dripping and how these methods affect the size, shape and biochemical performance of the laccase-alginate beads for further industrial ap-plication of lacease. The following variables were measured to parameterize the size and shape of the beads: the Sauter mean diameter (SMD), dry mass, particle-size distribution, aspect ratio (AR) and sphericity factor (SF). In addition, biochemical properties such as pH, temperature and thermal stability at different concentrations of alginate by assisted and not assisted extrude-dripping method were evaluated.
MATERIALS AND METHODS
REAGENTS
Sodium alginate and 2,2-azino-bis (3-ethylbenzothiazo-line-6) sulphonic acid (ABTS) were purchased from Sigma-Aldrich (Saint Louis, MO, USA) and CuSO4 from Carlo Erba (Milan, Italy). All other chemicals used were of ana-lytical grade.
PHYSICAL PROPERTIES OF ALGINATE SOLUTION
The density of the alginate solution was measured at room temperature using a 10 mL single-limbed pyenometer. Solution viscosity was determined by a viscometer (Brookfield viscometer DV-E, USA) in a range of between 6 and 100 rpm using spindle No. 62 for 1% (w/v) alginate solution and spin-dle No. 63 for 3% (w/v) alginate solution. All measurements were run in triplícate.
LACCASE PRODUCTION
Lacease was produced by semisolid culture of Trametes pubescens using coffee husk as substrate. The culture was conducted in 1 L shake flask with 50 mL of a basal médium (composition per liter: 0.5 g glucose, 2 g KH2P04, 0.25 g MgS04 -7HO, 0.9 g (NH4)2S04, 0.1 g CaCl2 and 0.5 g 15 KCl, in a citrate buffer 20 mM, pH 4.5) supplemented with 0.5 g L thiamine and 15 g of sterilized coffee husk. The culture was inoculated with three 10-mm plugs from active fungus cultured in malt extract agar.
After the culture was incubated for 19 days at 30°C without agitation, the enzymatic crude extract was removed by filtration through paper Whatman No. 1.
PRODUCTION OF ALGINATE BEADS
Beads with immobilized laccase were prepared by add-ing crude laccase (224 U/L) into sonicated 1.0% and 3.0% w/v sodium alginate solution at an enzyme-alginate ratio of 1:5 v/v. The mixture was mixed thoroughly for 60 min at room temperature to ensure homogeneous mixing. Then, the solution was extruded using a syringe (internal diameter 0.6 mm), assisted or manually (non-assisted), into 0.15 M CuS04 aqueous solution (pH 4.0) under magnetic stirring. The distance between the syringe needle and the gelling solution was fixed at 7.5 cm. The beads were left in the solution for 30 min in order for them to harden and were then washed exhaustively with distilled water. The formed beads were stored at 4 °C.
ASSISTED PRODUCTION OF ALGINATE BEADS
The assisted method was carried out using a programmable syringe pump fabricated by our research team. The syringe pump was made up by a stepper-rotor fed by a power circuit that was controlled by a computer program using a parallel port configuration. The stepper-rotor moved an end-less screw with a stopper that pushed the syringe backend. The pump was designed and built to be capable of pumping from low to médium high-density compounds. This attribute allowed the pump to drop compounds such as water or 3.0% w/v alginate without pressure differences. The syringe flow rate was set at 27.7 ul/min and controlled through a Matlab v. 7.12.0.635 (R2011a) interface.
SIZE ANALYSIS
Beads were dried at 20°C until constant weight was achieved and then measured to determínate the dry mass. The particle-size distribution was expressed as the relative standard deviation of the dry mass.
The Sauter mean diameter (SMD) was used to estímate the mean size of the beads (Chan, Lim, Ravindra, Mansa & Islam, 2009). It is defined as the diameter of a sphere that has the same volume to surface área ratio as the particle of interest. It is denoted according to Eq. 1 :

Where and indicates the volume and surface diameters respectively. The beads were observed under an optical microscope (Olympus CX21, Japan) and images were taken using a digital camera (Sony DSC-W350, Japan). All measurements were performed with 15 replicates.
SHAPE ANALYSIS
The sphericity factor (SF) and aspect ratio (AR) were used to estímate the roundness of the beads as described by Chan, Lim, Ravindra, Mansa & Islam (2012).The SF was calculated according to Eq. 2 :

Where and are the máximum and minimum diameters of the bead respectively. This valué varies from 0 for a completely symmetrical bead around its center to approaching the unit for an unshapely bead. The AR was computed as the máximum to minimum diameter ratio as shown Eq. 3 :

The AR is 1 for a symmetrical bead and it increases as the bead becomes more elongated. The SF and AR were calcu-lated using a population of at least 15 beads for each case.
ANALYTICAL DETERMINATIONS
Lacease activity was determined spectrophotometrically with ABTS (e420 = 36 M /cm) as substrate at room tempe-rature. One unit (U) was defined as |imol of oxidized ABTS by lacease per minute. The activities were expressed in U/L.
BlOCHEMICAL CHARACTERIZATION
The temperature and pH effect on lacease activity was estimated using ABTS as substrate. Optimal temperature was determined within the 40 - 70°C range, and optimum pH was measured over a range of 2.5 - 7 at its optimal temperature valué. Bead stability was tested at optimal pH and tempera-ture every two hours for 8 hours, and a final measurement at 24 hours. All measurements were run in triplícate.
STATISTICAL ANALYSIS
The main effect of the extruding method and sodium alginate concentration on the properties of the beads was evaluated using a full factorial design with 15 replicates through Mini-tab v. 15.1.20.0 (2007). Statistical differences were conside red for p< 0.05.
RESULT AND DISCUSSION
PHYSICAL PROPERTIES OF ALGINATE SOLUTION
The physical properties of alginate can vary widely, even when they originate from the same source, therefore, the alginate used in this study was characterized (Data not shown). The density of the alginate solution grew slightly as alginate concentration was increased. Additionally, the viscosity exhibited a large increase as alginate concentration rose. These results are in agreement with previous studies (Del Gaudio, Colombo, Colombo, Russo & Sonvico, 2005; Watanabe, Matsuyama & Yamamoto, 2012). As the shear rate increased, the 3.0% solution viscosity decreased, whereas the 1% solution remained invariant. Therefore, the 3.0% alginate solution is shear rate dependent and behaved like a non-Newtonian fluid within 6 to 100 rpm shear rates, whereas 1.0% alginate behaved like a Newtonian fluid.
SlZE ANALYSIS
Alginate beads with and without immobilized lacease were fabricated as described above. The dry mass measured for beads at 1.0% and 3.0% (w/v) alginate concentration by as-sisted and non-assisted extrude-dripping method is shown in Table 1 . As expected, 1.0% beads were lighter than those at 3.0% for both extruding methods. In this case, the rise in polymer content was mainly responsible for the observed increase in mass.
When methods were compared, it was observed that the extruding process affected the beads' dry mass regardless of bead formulation ( p < 0.05). As is shown in Table 1 , 1.0% assisted beads were heavier than 1.0% non-assisted beads; conversely, 3.0% assisted beads were lighter than 3.0% non-assisted beads. These differences are attributed to the varia-tions in the shear rate for extrusión, which had a direct effect on the rheological properties of alginate solution (Chan, et al., 2009). Beads prepared through assisted extrusión had a narrow size distribution compared with those produced through non-assisted extrusión for all compositions. The dispersión of 1.0% laccase-alginate, 3.0% laccase-alginate and 3.0% alginate beads by non-assisted extrusión were 10, 3 and 2 folds higher than those at the same composition by assisted method, respectively. While dispersión of 1.0% assisted beads was slightly lower than 1.0% non-assisted beads. The decrease in relative standard deviation (RSD) was due to the removal of the random error related to the shear rate for extrusión. When the syringe pump was employed, the shear rate was controllable and was fixed at 27.7 ul/min. In con-trast, when extrusión was handled manually, it was not pos-sible to apply an invariant shear rate in each repetition.
As is shown in Table 1 , the beads fabricated by assisted extrusión with and without lacease show significantly lower variations of the Sauter mean diameter (SMD) in compari-son to the non-assisted process. Regarding the non-assisted extrusión, the 3.0% laccase-alginate were the largest beads with a SMD of 2.81 mm; whereas, 1.0% alginate produced the smallest beads with a SMD of 0.65 mm. In brief, the latter beads were 4 times smaller than the 3.0% laccase-alginate beads. The variability between beads at the same alginate concentration was 83% and 84% for 1.0% and 3.0%, respectively. On the other hand, when beads fabricated with assisted extrusión were compared, the smallest beads (SMD = 0.52 mm) were 1.4 folds smaller than the largest beads (SMD = 0.73 mm). Furthermore, the variability between beads at the same alginate concentration was of only 0.8% and 3% for 1.0% and 3.0% beads, respectively.
In the case of the assisted extrusión, 3.0% beads were larger than 1.0% beads. As the shear rate was held constant, these size differences are explained by variations in viscos-ity and surface tensión of the solution (Park, Kim, Hwang, Kwon, Park, Choi, Yun & Kim, 2012). At higher alginate concentrations, the solution surface tensión increases and fluid becomes more viscous (Del Gaudio, et al., 2012; Wata-nabe, Matsuyama &Yamamoto 2003). Thus, a 3.0% alginate drop suspended at the needle tip grows for longer than a 1.0% alginate drop because of its higher resistance to break up. Consequently, more polymer detaches from the solution and the drop becomes larger. In fact, this effect also contributes to the rising of the dry mass. Similarly, adding lacease to alginate solution decreases the alginate concentration; therefore, viscosity decreases and less polymer is detached from the solution. As a consequence, the alginate-laccase beads pre-pared by assisted extrusión were smaller than beads without immobilized lacease, as shown in Table 1 .
Table 1. Size parameters prepared by assisted and non-assisted extrude-dripping methodSHAPE ANALYSIS
Dimensionless shape indicators are shown in Table 2 . Overall results reflect that roundness differs significantly when either the alginate concentration method or the extruding method is used. Statistically, the extruding method had no effect on the roundness of the beads ( p < 0.05). Alginate beads could be considered spherical, if spherical factor (SF) or aspect ratio (AR) is less than 0.05 and 1.1, respectively (Chan, et al., 2009). Therefore, only 3.0% assisted and non-assisted laccase-alginate beads, as well as 3.0% assisted alginate beads were considered spherical.
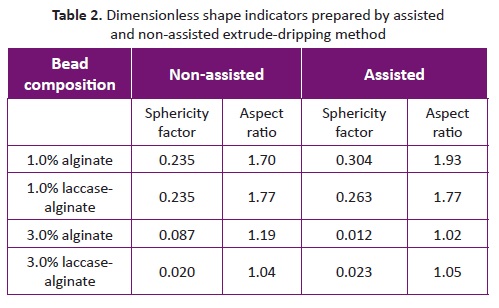
These results were consistent with previous studies (Del Gaudio, et al., 2012; Watanabe, Matsuyama &Yamamoto, 2003), which reported that the viscosity threshold necessary to enable the formation of spherical beads is above 60 - 50 mPa s. Besides, Chan (2011). found that the minimum alginate concentration to achieve an SF of below 0.05 is 1.5% w/v or an equivalent 130 mPa s. Nevertheless, 3.0% alginate beads by non-assisted extrusión were an exception. These results could be explained by the bead-size distribution. The shape of alginate beads is affected by the impact between the alginate drop and the gelling bath (Chan, 2011). Indeed, the degree of the impact is momentum dependent. For that reason, a larger drop or a higher distance between the needle and the gelling solution increase the momentum of the bead. As the distance was fixed at 7.5 cm for this study, the SF for 3.0% non-assisted beads is explained by its wider size distribution. Although, the extruding method had no direct effect on the bead shape, it could have an indirect influence through the dispersión of the size distribution.
PH AND TEMPERATURE STABILITY OF FREE AND IMMOBILIZED LACCASE ON ALGINATE BEADS
Temperature and pH effect on free and immobilized lacease activity were evaluated individually using ABTS as substrate. The relative activity profiles determined for laccase-alginate beads by assisted and non-assisted extrude-dripping methods are shown in Figure 1 and 2 . In general, beads presented similar performance with their maximal between 50 and 55°C and a pH range of 2.0 to 3.0. The relative activity for all compositions was below the free lacease profile, with the exception of the máximums of assisted profiles. This decrease in the activity is related to internal diffusive phenomena, which restriets the total expression of lacease activity. In addition, it was observed for temperature profiles that the total activity of beads (the área under the curve) by assisted extrusión is higher compared to non-assisted extrusión, regardless of the alginate concentration.
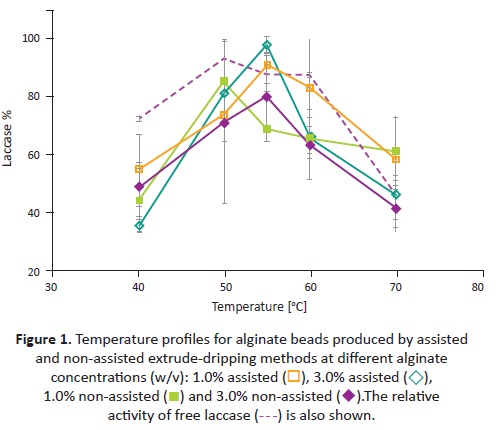
Free lacease and 1.0% non-assisted presented the máximum relative activity at 50°C, and 3.0% non-assisted along with 1.0% and 3.0% assisted presented their máximums at 55°C. Henee, the máximum relative activity shifted positively by 5°C, compared to the free lacease máximum, when the en-zyme was immobilized in alginate by assisted extrusión. For the assisted method, the profiles behaved similarly with an activity decrease at 40°C, a peak at 55°C and a subsequent activity loss. For the non-assisted method, temperature profiles for different alginate concentrations did not present congruence.
In general the pH profiles (Figure 2 ) behaved compara-tively, with the maximal acid valúes followed by a decrease in activity as pH increases. The highest relative activity for free lacease was obtained in a fíat zone between pH 2.0 and 4.0; in comparison, the máximum activity for immobilized laceases was at either pH 2.0, 2.5 or 3.0. The displacement of the máximum in pH profiles is due to the H+ absorption capacity of charged supports like alginate. This explained the shift of the máximum relative activity of 1.0% assisted beads and non-assisted beads at both alginate concentrations to more alkaline valúes. These results coincided with those found in other studies (Lu, et al., 2007).
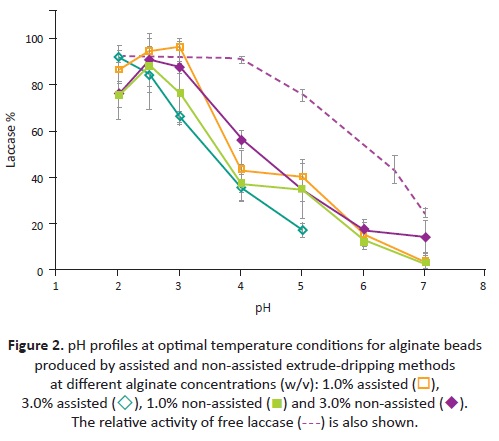
The major variability in the measurements of the beads' activity was observed when extrusión was handled manually. The standard deviation for assisted extrusión was below 20%, while for non-assisted extrusión, it varied from 2% to 80%. The broader variations through non-assisted extrusión could be attributed to the wider size distribution.
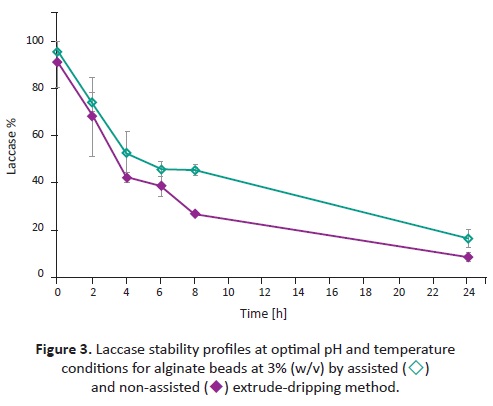
LACCASE STABILITY
Beads produced at 3.0% (w/v) alginate concentration were chosen for the stability analysis because they presented the lowest size distribution for both extruding methods. The highest relative activity with ABTS for 3.0% beads was around 55°C and pH 3.0; thus, these conditions were selected for the stability test. The progress of lacease activity during the stability test for 3.0% assisted and non-assisted beads are shown in Figure 3 . The half-life time, defined as the time that it takes for the relative activity to decrease by half, for the assisted and non-assisted profiles were 4.8 and 3.4 hours, respecti-vely. Lacease stability is important to evalúate its viability in enzyme reuse and its application in long-term reactions.
CONCLUSIONS
The extruding method employed for bead-production affeets the bead-size and size distribution. Depending on the extruding technique ( p < 0.05), there was a significant effect on the dry mass of the beads. In addition, it was shown that the SMD beads were more homogeneous when the assisted extrusión method was employed. The size distribution-RSD-of 1-0% laccase-alginate beads was 29.2% and 7.8% for beads fabricated through non-assisted and assisted extrusión res-pectively. Equally, the RSD of 3.0% laccase-alginate beads by assisted extrusión was 3 folds lower than for those at non-assisted extrusión. Therefore, RSD was narrower for assisted extrusión than for non-assisted extrusión for all composition. The effect of the extruding method on the bead-size was at-tributed to shear rate variations, which had a direct effect on the rheological properties of alginate solution. Consequently, the assisted method is preferred as it allows the production of more statistically homogeneous lacease particulate material for large-scale production.
SF and AR Índices show that bead-shape statistically depends on alginate solution properties inherent to alginate concentration instead of the extruding method. Furthermore, spherical beads were achieved exclusively when the high-est alginate concentration was employed. These results are consistent with previous studies by Chan, et al., 2009; Del Gaudio, et al, 2012; Chan (2009) 3.0% non-assisted beads were not considered spherical; therefore, shape was indirectly affected by the extruding method through the degree of dispersión of the size distribution.
Biochemical characterization revealed an optimal temperature of around 55°C and an optimal pH of between 3 - 4 for both extruding methods and alginate concentration. Similar performance for free and immobilized lacease was obtained previously (Lu, et al., 2007; Gaitan, Medina, González, Rodríguez, Espejo, Osma, Sarria, Almeciga-Diaz, Sánchez 2011). Differences in the profile tendencies became evident when they were compared among methods. In addition, higher variability in the measurements was obtained for non-assisted beads. Assisted beads showed higher enzymatic stability at optimal conditions. Thus, the use of the assisted extrusión methods is highly recommended for eyelie or long-time bioprocesses as the Ufe eyele of the encapsulated enzyme can be increased by about 40%. In addition, a more homogeneous encapsulated catalytic material is produced due to a slow fatigue curve from electromechanical sys-tems compared to manual ones. In general, bioprocesses are enhanced by highly homogeneous particulate material easing temperature or pH controlled steps; thus, an assisted method presents several benefits for producing large quantities of lacease particulate material.
ACKNOWLEDGEMENTS
The authors wish to dedicate this work to Professor Antonio García Rozo, founder and former director of CMUA, for his outstanding and encouraging career. Authors also want to thank Sara Segura and Mario Malagón for the fabrication and maintenance of the programmable syringe pump.
References
Chai, Y., Mei, L.H., Lin, D.Q., and Yao, S.J. (2004). Diffusion coefficients in intrahollow calcium alginate microcapsules. Journal of Chemical and Engineering Data, 49, (3), 475-478. [ Links ]
Chan, E.S.. Lee, B.B., Ravindra, R and Poncelet., D. (2009). Prediction models for shape and size of ca-alginate macrobeads produced through extrusion-dripping method". Journal of Colloid and Interface Science, 338 (1), 63-72. [ Links ]
Chan, E.S., Lim, T.K., Ravindra, R, Mansa R.F., and Islam, A. (2009). The effect of low air-to-liquid mass flow rate ratios on the size, size distribution and shape of calcium alginate particles produced using the atomization method. Journal of Food Engineering, 108(2), 297-303. [ Links ]
Chan, E.S. (2011). Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficieney and bead properties. Carbohy-drate Polymers, 84 (4), 1267-1275. [ Links ]
Del Gaudio, R, Colombo, R, Colombo, G., Russo R, and Sonvico, F. (2005). Mechanisms of formation and disintegration of alginate beads obtained by prilling. International Journal of Pharmaceutics, 302, (1-2) 1-9. [ Links ]
Dominguez, A. Gómez, J. Lorenzo, M., and Sanroman., A. (2007). Enhanced production of lacease activity by Trametes versicolor immobilized into alginate beads by the addition of different inducers. World Journal of Microbiology & Biotechnology, 23 (3), 367-373. [ Links ]
Faraco, V., Pezzella, C, Miele, A. Giardina R, and Sannia, G., (2009). Bio-remediation of colored industrial wastewaters by the white-rot fungi Phanerochaete chrysos-porium and Pleurotus ostreatus and their enzymes. Bio-degradation, 20(2), 209-220. [ Links ]
Fernández-Fernández, M., Sanromán, M.Á., and Moldes, D. (2012). Recent developments and applications of immobilized lacease. Biotechnology Advances, 31(8), 1808-1825, doi: 10.1016/j.biotechadv.2012.02.013. [ Links ]
Gaitan, I.J. Medina, S.C.. González, J.C., Rodríguez, A., Espejo, A.J., Osma, J.F. Sarria, V., Almeciga-Diaz, C.J., and Sánchez, O.F. (2011). Evaluation of toxicity and degradation of a chlorophenol mixture by the lacease produced by Trametes pubescens. Bioresource Technology, 102 (3), 3632-3635. [ Links ]
Lu, L., Zhao, M., and Wang, Y.(2007).Immobilization of lacease by alginate-chitosan microcapsules and its use in dye decolorization. World Journal of Microbiology & Biotechnology, (23) 2, 159-166. [ Links ]
Mohidem, N.A., and Bin Mat, H.. (2012). Catalytic activity and stability of lacease entrapped in sol-gel silica with additives. Journal of Sol-Gel Science and Technology, 61, (1) , 96-103. [ Links ]
Niladevi, K.N., and Prema, P. (2008). Immobilization of lacease from Streptomyces psammoticus and its application in phenol removal using packed bed reactor. World Journal of Microbiology & Biotechnology, 24 (7), 1215-1222. [ Links ]
Osma, J.F., Toca-Herrera, J.L., and Rodriguez-Couto, S. (2011) Cost analysis in lacease production. Journal of Environmental Management, 92 (11), 2907-2912. [ Links ]
Park, H., Kim, P.-H., Hwang, T., Kwon, O.-J., Park, T.-J., Choi, S.-W., Yun, C.-O.and Kim, J.H. 2012. Fabrication of cross-linked alginate beads using electrospraying for adenovirus delivery. International Journal of Pharma-ceutics, 427(2) , 417-425. [ Links ]
Radha, K.V. Regupathi, I., Arunagiri, A., and Murugesan, T.(2005). Decolorization studies of synthetic dyes using Phanerochaete chrysosporium and their kinetics. Process Biochemistry, 40(10) 3337-3345. [ Links ]
Shilpa, A., Agrawal, S.S., and Ray, A.R.. (2003).Controlled delivery of drugs from alginate matrix". Journal of Ma-cromolecular Science Polymer Reviews, C43 (2),187-221. [ Links ]
Watanabe, H. Matsuyama, T.and Yamamoto, H. (2003). Experimental study on electrostatic atomization of highly viscous liquids. Journal of Electrostatics, 57 (2)183-197. [ Links ]













