Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Med
Print version ISSN 0121-5256
rev.fac.med vol.21 no.2 Bogotá July/Dec. 2013
ARTÍCULO DE REVISIÓN
INFLUENZA H5N1 - NEXT PANDEMIC?
INFLUENZA H5N1 - PRÓXIMA PANDEMIA?
INFLUENZA H5N1 - PANDEMIA PRÓXIMO?
JEFFERSON GUERREROa, LINDA NAVARRETEb, DIANA ROLDÁNa,MARÍA PAULA PEÑAb, JEIMY OVIEDOb, IVÁN MÉNDEZc
a Medical student, School of Medicine, Universidad Militar Nueva Granada.b Medical student, School of Medicine, Fundación Universitaria Juan N. Corpas.
c Magister, Pathogenicity group Research, Professor of Infectious diseases, Universidad Militar Nueva Granada and Fundación Universitaria Juan N. Corpas.
* Correspondencia: ivan.mendez@unimilitar.edu.co
Recibido: Abril 3 de 2013 Aceptado: Julio 27 de 2013
Abstract
Since 1990 there have been cases of patients infected by influenza caused by Low Pathogenic Avian Influenza. However, since 1997, highly pathogenic viruses, such as A/H5N1, have caused an increase in the number of people infected via zoonotic transmission. Infected patients have constitutional and mainly respiratory symptoms, hematological disorders and liver dysfunction. About half of patients are under 20 years and about 90% under 40 years. In recent studies in mammals it seems that only few mutations in viral genome are necessary to confer the virus the ability to infect mammalian cells. In response to these studies, the Centers for Disease Control (CDC) propose continuous researches without alerting the health care system, because, at present, there is not a global threat. Moreover, in the future, this issue would take place as a major concern from the point of view of public health; it could be the next pandemic. Due to the high human mortality rate occurring in cases of patients infected with this virus, an outbreak or pandemic would require proper knowledge of the biology of the avian influenza virus, the determinants of pathogenicity, prevention through vaccination and possible new treatment regimens.
Key words: Influenza, transmission, pandemics, H5N1 subtype.
Resumen
Desde 1990 se han reportado casos de pacientes infectados por influenza causadas por virus de baja patogenicidad, sin embargo, desde el año 1997, virus altamente patógenos como el A/H5N1 han provocado un aumento en el número de personas infectadas por transmisión zoonotica. Los pacientes infectados presentan síntomas constitucionales y respiratorios, trastornos hematológicos y disfunción hepática. Cerca de la mitad de casos son pacientes menores de 20 años, y aproximadamente el 90% son menores de 40 años. Recientes estudios en mamíferos indican que son necesarias algunas mutaciones en el genoma viral para que el virus tenga la capacidad de infectar células mamíferas. En respuesta a estos estudios el centro de control de enfermedades (por sus siglas en ingles Centers for Disease Control), ente regulador en la dinámica epidemiológica mundial propone continuar con las investigaciones sin alertar a los sistemas de salud, pues en este momento no constituye una amenaza mundial. Sin embargo, este aspecto toma lugar como una preocupación importante desde el punto de vista de la salud pública, por cuanto podría constituir la próxima pandemia. Debido a la alta mortalidad en humanos que se presenta en los casos de pacientes infectados con este virus, un brote o una pandemia exigirán por tanto un conocimiento apropiado de la biología del virus de la influenza aviar, los determinantes de su patogenicidad, la prevención mediante vacuna y los posibles esquemas de tratamiento.
Palabras clave: Influenza, transmisión, pandemias, subtipo H5N1.
Resumo
Desde 1990 se têm reportado casos de pacientes infectados por influenza causadas por vírus de baixa patogenicidade, porém, desde o ano 1997, vírus altamente patógenos como o A/H5N1 têm provocado um aumento no número de pessoas infectadas por transmissão zoonótica. Os pacientes infectados apresentam sintomas constitucionais e respiratórios, transtornos hematológicos e disfunção hepática. Cerca dametade de casos são pacientes menores de 20 anos, e aproximadamente o 90% são menores de 40 anos. Recentes estudos em mamíferos indicam que são necessárias algumas mutações no genoma viral para que o vírustenha a capacidade de infectar células mamíferas. Em resposta a estes estudos o centro de controle de doenças (por suas siglas em inglês: Centers for Disease Controle), ente regulador na dinâmica epidemiológica mundial propõe continuar com as pesquisassem alertar aos sistemas de saúde, pois em este momento não constituiu maameaça mundial. Porém, este aspecto toma lugar como uma preocupação importante desde o ponto de vista dasaúde pública, já que poderia constituir a próxima pandemia. Devido à alta mortalidade em humanos que se apresenta nos casos de pacientes infectados com este vírus, um surto ou uma pandemia, exigirão por tanto um conhecimento apropriado da biologia do vírus da influenza aviar, os determinantes dasua patogenicidade, a prevenção mediante vacinae os possíveis esquemas de tratamento.
Palavraschave: Influenza, transmissão, pandemias, subtipo H5N1
Introduction
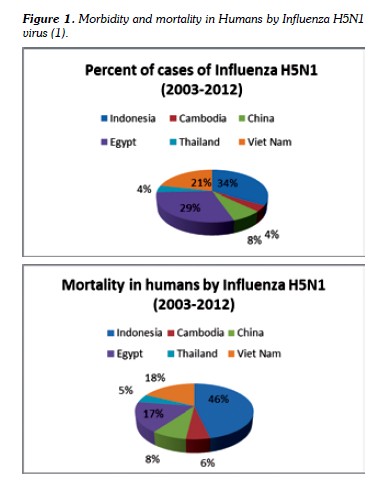
Since 2003, the World Health Organization (WHO) reported about 600 human infections by H5N1 influenza virus in 15 countries in Asia, Africa, Europe and the Near East, with an associated mortality rate approaching 60% (2).
In 2011, there were 62 cases and 34 deaths in humans attributable to H5N1 in five countries (Bangladesh, Cambodia, China, Egypt and Indonesia) and recent outbreaks have occurred in poultry in Bangladesh, China, Egypt, India, Indonesia and Vietnam (1,3).
Antigenic variation in surface glycoproteins of the virus, known as Hemagglutinin HA and Neuraminidase NA, are responsible for adaptability to hosts other than birds. Infected people start the clinical picture with high fever and presence of lower respiratory tract symptoms. Some patients have diarrhea, vomiting, abdominal pain, pleuritic pain and nosebleeds. The early detection of H5N1 flu cases will be critical for the proper monitoring of the pandemic, treatment of patients, isolation measures to reduce transmission and introduction of immunoprophylaxis (2,4).
The aim of this review is to acquire knowledge about the past, present and future of Influenza H5N1 virus and possible pandemic in light of the latest scientific advances in the field of influenza virus.
Epidemiology
The influenza virus has well known animal reservoirs such as pigs, horses, sea mammals and birds; however the latter are considered the natural reservoir of all subtypes, and although the incidence of human infection by avian virus is low, since 1997 the possibility of transmitting avian influenza to humans has been recognized, however, no studies of seroprevalence have been conducted to recognize the reality facing of avian influenza H5N1 strains in humans (5-8).
The Report of the World Health Organization, in January 2013, recorded human infections attributable to H5N1 from 2003 to January 2013 as follows: 610 cases with 360 deaths (59%), Figure 1 (1,9).
It is well known to mankind the impact of influenza virus throughout many epidemics, especially caused by H2N2 strain in 1957, H3N2 in 1968, H1N1 in 1917 and 2009, and in animals with human transmission of H5N1 in 1997 and H9N2 in 1999 (5,10-12).
The first reported case of human infection (May 1997- Hong Kong) with influenza A H5N1 was in a 3 year old boy who presented odynophagia, fever and cough, being recorded a total of 18 cases that year, re-emerging in 2003 by a different strain; 11 patients between 1998 and 1999 were infected with H9N2 with benign clinical course than produced in 1997 by H5N1. Other viruses such as H1N2 and H7N7 have been associated with human conjunctivitis and flu symptoms, but with low mortality rate (5,12,13).
In 2004, the influenza A H5N1 virus affected eight countries with a mortality near 80%, where every patient involved had close contact with poultry. The Ungchusak study published in 2005 using RT-PCR (retro-transcriptase polimerase chain reaction) reported the eventual person to person transmission of this virus and warned about the possibility that a new variant that can be transmitted human to human could be generated through mutations or gene rearrangements. This virus H5N1/2004 is antigenically different from the 1997 strain, indicating that this variant arose in a short time adapting to humans by mutation or recombination (12,14-16).
It is important to keep in mind that mankind has transcended four pandemics of influenza virus. The possibility that influenza virus strains, as the case of A H1N1/2009 and others, have a deep impact worldwide is certain; the Centers for Disease Control (CDC) stated at that time that implementation of a vaccine against this viral strain is essential as well as considering it for other types such as H5N1 virus (5,17,18).
Etiology
Avian flu is an infectious disease caused by an Orthomyxoviridae (Greek orthos: 'standard, right', and myxo: 'mucus') virus, known as influenza virus type A (19,20,21). The virus is a single-stranded RNA of negative polarity and pleomorphic appearance, with an average diameter of 120 nm which replicates with no mechanism of RNA protection, being frequent the alterations in genome. An unique feature of these viruses is that they are segmented. The virus adheres to the cells easily through a surface glycoprotein known as hemagglutinin (HA), from which 16 different types are known (5,18,22). Another feature becomes to its ability to leave the infected cell due to the surface glycoprotein neuraminidase (NA - 9 types are known). There are 144 possible combinations of influenza virus type A (21,23). Furthermore, it is noteworthy that the virus must meet three requirements to be considered with pandemic potential: enter and replicate in the human body, cause disease, and easily be transmitted from person to person.
Studies have shown that there are two clinical forms of avian flu. The mild form is produced by low pathogenic strains designated LPAI (Low Pathogenic Avian Influenza), while lethal forms are caused by highly pathogenic strains or HPAI (High Pathogenic Avian Influenza). The difference between them can be set initially by subtyping; the only subtypes showing HPAI pattern are H5 and H7. Besides subtyping, it is necessary to demonstrate in vivo the lethality of the strains in a laboratory assay in which 4 to 6-week old chickens are inoculated intravenously; if resulting mortality rate is 75% or more, the strain is considered HPAI.
The virus has many proteins such as polymerase, hemagglutinin (HA), nucleocapsid protein (NP), neuraminidase (NA), and matrix protein (M) as M2 which acts as an ion channel maintaining stable pH of the endosome and nonstructural proteins (NS). The activity of the RNA polymerase, which is formed by PB2, PB1 and PA, is responsible for replication and transcription. This enzyme has an endonuclease activity and is linked to RiboNucleo Protein: RNP. The NS1 and NS2 proteins have regulatory function to promote the synthesis of viral components in the infected cell. Hemagglutinin (HA) is a glycoprotein which contains 2 to 3 glycosylation sites and serves as a receptor to sialic acid (N-acetyl-neuraminic acid) and induces penetration of the viral particles by fusion to host cell membrane. HA is the major antigenic site of influenza virus. Mutations in this protein reduce or inhibit the binding of neutralizing antibodies, emerging new subtypes through two phenomena known as antigenic drift and antigenic shift. Antigenic drift is the explanation of seasonal epidemics but antigenic shift, which is called genomic rearrangement, occurs for example when H1 is replaced by H5 and results in the formation of a mosaic virus; this can occur when the cell is infected with two different viruses at the same time and their genomic segments are exchanged during replication.
In humans, influenza strains preferentially recognize receptors formed by oligosaccharid Siaα2,6Gal. On the other hand, avian and equine influenza strains preferentially recognize the receptor Siaα2,3Gal. Consequently, influenza strains infect tissues in various species as a function of the presence or predominance of these types of receptors. Human respiratory epithelial cells contain predominantly Siaα2,6Gal while airway of horses and intestinal tract of ducks (where avian strains replicate) contain mostly remains of Siaα2,3Gal. Interestingly, the respiratory tract of pigs contain both types of receptors, which explains their susceptibility both to avian and human strains (20,21,24,25).
A major factor that determine the susceptibility of host is the presence of these sialic residues in different cells and tissues of each animal species, since all avian strains have Glu (glutamic acid), whereas all human strains (H1N1, H2N2 and H3N2) possess Lys (lysine). Therefore, the appearance of Lys in HA of an avian strain is considered an adaptive mutation to mammalian cells. So far, they have only been detected in avian strains isolated from infected human patients. The other major glycoprotein, neuraminidase (NA), catalyzes the glycosidic bonds with sialic acid, action involved in the release of virions from infected cells. The antigenic drift can occur in the NA and it may determine resistance to NA inhibitors such as oseltamivir and zanamivir.
Studies by Li et al have shown that about 50% of the H5N1 strains recovered post-infection from the mouse lungs had the substitution of Glu for Lys. Furthermore, mutations at position 701 of PB2 gene seem to determine or facilitate the ability of avian influenza strains to cross the species barrier and replicate in mammals. The acquisition of an Asn (asparagine) instead of Asp (aspartic acid) (Asp701Asn) was already observed in avian H7N7 strains adapted to mouse lung cell lines and mouse-adapted human strains. The most important aspect is that mutation Asp701Asn could normally occur spontaneously in H5N1 influenza strains, facilitating the transmission of these strains to mammals (5,26).
Pathophysiology
Infection involves transfer of respiratory secretions or indirect contact (fomites) from an infected individual to another. It is essential to remember that H5N1 virus has a high survivability in the environment (26).
The incubation period is 2-5 days, although other studies indicate periods of up to eight days It is important to analyze patients who have traveled to endemic areas 7-14 days prior to symptoms onset or those with close contact with sick travelers from endemic countries.
Once the virus is in respiratory tract epithelium, it may adhere and penetrate cells giving rise to viral replication (12).
As mentioned before the potential pandemic viruses have the following features: replicate in the human body, cause disease, and are easily transmitted from person to person. The literature indicates that the influenza virus with the greatest pandemic potential today is the H5N1 subtype, which has met the first two conditions.
Due to the presence of changes in the two major surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), the influenza virus is able to elude the immune response (5).
The influenza virus binds to the cell by HA interaction with a glycoprotein receptor on the cell surface, as previously described. Only the hemagglutinin is recognized by trypsin, a respiratory and digestive tract protein of the host cells, allowing the invasion of these tissues and explaining why HA antigen is a key protein responsible for adherence to susceptible cells (12,27).
The entry of the virus occurs by endocytosis, where merging with a lysosome occurs to causing decrease in pH and structural changes in haemagglutinin. Later, it will interact with the nuclear membrane thus being able to enter the nucleus of the viral genome. In the same way, some nucleoproteins have affinity for a series of intermediates which interact with the nuclear pore complex allowing access to the nucleus. This only occurs if these nucleoproteins are free and not associated with matrix proteins (28).
Polymerase begins the transcription of viral Messenger RNA using this process for translation of early viral proteins NP and NS1, and then translation of the host messenger RNA is blocked. It generates an accumulation of NP thus triggering viral RNA synthesis. Secondary transcription generates late translation proteins such as M1, HA and NA; the last two are assembled in the cytoplasm and transported to surface where are integrated into cellular membrane. The nucleocapsid is enclosed by M proteins, and viral particles leave the cell through the cell membrane with their envelope together with surface glycoproteins (7,29).
The disease is originated from the immune system and caused in response to infection by the release of cytokines and endocrine activation (30,31).
Viruses particles are trapped by the mucociliary layer in the upper respiratory tract. If the viruses elude it, they reach the alveoli where macrophages or dendritic cells could destroy them.
In flu infection, cells of the innate immune system are activated by the presence of viral RNA; this activation is mediated by Toll Like Receptor 8 - TLR8.
The released cytokines and chemokines attract the effector cells of the immune response such as neutrophils, monocytes, plasmacytoid cells, and dendritic cells to the alveoli.
The major cytokines are IL-1, TNF-alpha, IL.6, IFN, IL-8, macrophage inflammatory protein (MIP-1) as well as acute phase proteins. The synthesis of acute phase proteins in liver is triggered by the presence of IL-6, glucocorticoids and catecholamines (31,32).
The viral infection induces interferon (IFN) release to protect from infection and to inhibit viral replication, but influenza virus has mechanisms which counteract IFN release. The NS1 protein acts as an antagonist, because it works as a scavenger to prevent detection of viral RNA (30,31).
The proinflammatory cytokine release causes an inflammatory process as well as airway hyperemia o bleeding with bronchial hyperreactivity and airway obstruction (31,33).
The innate immune response is intended to limit viral replication and adaptative immune response through antibodies to neutralize the surface proteins of the virus yet activation of Th1 lymphocyte is needed for a cytotoxic response that eliminates the virus in infected cells (31,34).
Clinical and pathological findings in humans
H5N1 virus replicates in the nasal or pharynx epithelium and then spreads to the mucous membranes of the respiratory system, but can also be spread throughout the body and cause the systemic form of the disease (26).
The clinical feature can range from asymptomatic infection, minimum alterations of upper and lower airways up to multiple organ impairment. Hepatic dysfunction and lymphopenia have been shown (12,35).
Sudden onset clinical manifestations include fever higher than 39°C, dry cough and rhinorrhea (lower respiratory tract symptoms) accompanied by fatigue, weakness, myalgia, arthralgia, headache, conjunctivitis, chest pain, dyspnea, pain abdominal, irritability, diarrhea, vomiting, pleuritic pain and nasal bleeding, Figure 2 (12).
Patient with severe influenza A H5N1 infection might progress through a pathogenic pathway distinct from that of the usual human subtypes H1-H3. Replication of influenza A H5N1 in the human respiratory tract might have a potential to trigger a stage of hypercytokinemia and complicated with hemophagocytic syndrome (36).
In the early days and while the number of people affected is small, patients with suspected influenza A H5N1 virus should be hospitalized in order to isolate them and to establish clinical monitoring, diagnosis and antiviral treatment (37).
Diagnosis
For case detection, the presence of cough and fever has good predictive value. The laboratory confirmation of infection with the H5N1 virus requires at least one of the following positive results: isolation of the virus, RT-PCR for H5N1 A virus RNA, immunofluorescence test for antigen detection using monoclonal antibodies against H5, and at least a 4-fold increase in specific antibody titers (38,39).
Documented case records indicate that chest radiography is essential in the initial examination. Chest X-ray may reveal bilateral interstitial infiltrate, lobar collapse, consolidation and air bronchogram without pleural effusion. However, none of these changes, either alone or together, is specific for influenza infection (7).
The major hematologic findings in H5N1 patients are anemia (hemoglobin <12 g/dL) in 30% of patients, leukopenia (WBC <4 x 109/L) in 60%, neutropenia (neutrophils <1.5 x 109/L) in 62.5%, lymphopenia (lymphocytes <1.5 x 109/L) in 50%, and 60% thrombocytopenia (platelets <150 x 109/L).
Within the parameters studied, it was found that anemia is not associated with mortality, whereas leukopenia, neutropenia, lymphopenia and thrombocytopenia if they were predictors of a poor prognosis (40).
Treatment
There are two antiviral classes available for treatment of H5N1: M2 inhibitors (amantadine and rimantadine), and neuraminidase inhibitors (zanamivir and oseltamivir). M2 inhibitors act by blocking the virus within the cell; they are only effective against influenza A virus, are associated with a number of toxic effects and resistance; moreover, this resistance is genetically stable and transmitted to susceptible contacts which limits their use (18).
Neuraminidase inhibitors interfere with the release of influenza virus progeny from infected cells, which prevents infection of new cells and reduces the spread of the virus in the respiratory system. Unlike M2 inhibitors, neuraminidase inhibitors exhibit less toxicity and induce less resistance, they are effective against all subtypes of neuroaminidasas and all types of influenza virus, including H5N1, however, the antigenic drift can occur in the NA and mutations can condition its use (18,28).
In patients infected in the 1997 outbreak, treatment with corticosteroids (methylprednisolone, 5 mg/kg/d) and antiviral agents (oseltamivir, 75 mg twice daily or ribavirin, 400 to 800 mg three times daily) seemed ineffective. Six out of seven patients receiving corticosteroids died and 80% of patients receiving oseltamivir died as well. Some patients required mechanical ventilation, especially in the first two days of the illness (41).
Genetic sequencing of the H5N1 subtype isolated in five patients in 2004 showed resistance to amantadine and rimantadine; moreover, susceptibility testing for oseltamivir has shown sensitivity of the virus to the drug (7).
For management of bacterial coinfection, especially with Staphylococcus aureus, early use of broad spectrum antibiotics is advisable (28).
Prevention
Vaccination is the primary strategy for preventing a pandemic as well as antiviral prophylaxis and epidemiological surveillance (42).
Some vaccines against H5 antigens have shown little immunogenicity and have required high doses of HA or the combination with the adjuvant MF59 or HT for generating a neutralizing antibody response. One of these vaccines is based on inactivated H5N1 strains isolated in 2004, which in practice can lead to the administration of two doses of vaccine. Vaccination is the primary strategy for preventing influenza. There are probably a number of scenarios in which other measures such as antivirals must be used, which strategically applied can have an important role in reducing the impact of the pandemic and slowing its spread (18,43).
The vaccine should be able to induce cross-protection against antigenic variants and, in addition, protection in the event that the virus causing the pandemic continues to mutate. For better performance and improved antigenic immune response, a pharmaceutical company has formulated its H5N1 vaccine with an adjuvant system called AS03. The AS03 adjuvant system consists of an emulsion of oil in water with a known immunomodulator, Vitamin E (alpha-tocopherol), in order to maximize the immunological benefits.
It is well established that water-oil emulsions can induce high antibody concentrations when mixed with a certain antigen, and trigger the secretion of cytokines, thereby enhancing the recruitment of cells of the innate immune system. A couple of vaccines have been developed to address the pandemic and a pre-pandemic scenario (Pandemrix®, Prepandrix®, GlaxoSmithKline Biologicals, Rixensart, Belgium) both approved in the European Union in May 2008 for active immunization against the A/H5N1 flu virus in adults aged between 18 and 60 years, and recently (May 2009) in adults over 60 years of age. The recommended dosage is 0.5 ml containing 3.75 mcg of HA administered at an interval of at least 21 days. Safety data include more than 10,000 subjects who received the AS03-adjuvanted H5N1 vaccine, mostly recruited in large studies conducted in Europe and Asia (16).
Discussion and conclusions
In late 2011, the scientific community had general knowledge of the findings from research in H5N1 influenza virus and its transmissibility among mammals associated with mutations in the HA gene. The receptor molecule for H5N1 avian influenza is the Siaα2,3Gal. In contrast, human cells from human airway expressed the Siaα2,6Gal (24).
The news sparked a broad debate about publishing openly or holding scientific information. In December 2011, the National Science Advisory Board for Biosecurity (NSABB-National Science Advisory Board for Biosecurity) independent advisory body to the Department of Health and Human Services of the United States-USA issued an alert through the newsletter of the National Institute of Health recommending that authors and publishers of Nature and Science journals avoid including the methodological details of the experiments, making reference to security reasons for the possible use of the information for purposes of bioterrorism (44).
The researchers conducted by Dr. Ron Fouchier of the Erasmus Institute in Holland and Dr. Yoshihiro Kawaoka of the University of Wisconsin, USA, included millions of variants of the H5N1 virus by reverse genetics, of which only one was able to recognize the human rather than the avian receptor. This variant had the following changes: glutamine by a leucine (Q226L) and an asparagine for a lysine (N224K); then they merged the HA mutated gene with H1N1 virus.
Ferrets (mammalian model of choice for studying influenza virus) were infected with H5N1-H1N1 hybrid virus. After six days, the researchers realized that they had acquired a third mutation, asparagine for an aspartic acid (N158D). After the researchers infected mice with the triple mutant strain they found that the virus could infect ferrets by airborne transmission in some nearby cages. Some of these viruses had a fourth mutation, a threonine for an isoleucine (T318I) (24,25).
None of the infected ferrets died; this strain called H5HA/H1N1 was sensitive to oseltamivir and when the rate of dispersion among these was measured, it was slower than that of H1N1 (25).
It is not clear whether the virus could spread among humans as it did among the ferrets. However, the four mutations are necessary for adhesion to human cells, three mutations alter the adhesion properties of the HA and the fourth stabilizes the receptor binding capacity (24,41), besides, the different values of body temperature could act as an enabling mechanism for Influenza virus infection through an adaptative mechanism of the virions in the species susceptible to infection, including human beings (45).
Prior to the publication of the first of two researches over H5N1, the debate was opened between scientists and bureaucrats about the impact of the research results. The first who made his contribution to the discussion was one of the group leaders, Dr. Kawaoka, indicating that the paper was aimed at containing the risk of the possible spread of H5N1 between humans; however in January 2012 he declared a moratorium of 60 days indicating the urgency to continue working with the virus and publication the results (2).
Simultaneously, the group of Dr. Fouchier joined the 60-day pause in working with both the H5N1 virus in the laboratory and on animals (46). In an interview published in Science, Dr. Fouchier said that in addition to identifying particular mutations, the most striking aims to establish the mechanism by which the influenza virus infects cells; he added that manipulation and generation of mutant strains with potential for airborne transmission among mammals is not easy (47,48).
In April 2012, the discussion was extended to the funding, control and monitoring of research projects representing potentially harmful effects on human health (49).
The coexistence of circulating influenza viruses has given evidence on the evolutionary biology of these microorganisms. The AH1N1/2009 variant proved to be a recombinant strain of avian, human and swine influenza virus; experimental evidence of work with H5N1 shortened a path of adaptability that would occur naturally between strains. Here it is important to recall that the human immunodeficiency virus emerged from a strain of primate origin.
The influenza virus is known for its genetic variants particularly in the HA and NA proteins, processes that are closely related to both mutations as recombinant process between circulating variants. Its seasonal pattern and pandemic potential in 1889, 1918, 1957, 1968 and 2009 is also remarkable. Kawaoka mentioned that it is relevant for humanity to recognize these possible variations, in particular those involving risk of pandemic (2).
It is of interest not only the recognition of these variants to prepare control measures but also the isolation methods, infection model, and drug resistance until vaccine development. Although the information could be considered risky because of its potential use in the construction of a biological weapon, it is also known that generation of these mutant strains is highly demanding in laboratory conditions.
The knowledge regarding the biology and impact of influenza viruses has been recognized by humanity over time throughout pandemics of the twentieth century, likewise the development of vaccines for seasonal and non-seasonal virus and emergence of oseltalmivir resistance (50,51). For that reason, new information about this virus should be considered not only of scientific interest but also of interest to public health, so there should be no restriction. Governments should consider using this information to protect their populations from the impact of changes that could occur naturally in the biology of influenza virus even without any direct human intervention.
Although current H3N2 or H1N1 vaccines may protect against a virus similar to H5HA/H1N1, the continuous evolution of influenza reinforces the need to prepare and update the vaccines to all possible variants, others bird flu virus serotypes like H7N9, H5N2 and H10N8 have been described recently in human infections for that reason is overriding strengthen basic and applied research allowing elucidating particular aspects of influenza virus transmissibility yet going one step ahead of this prevalent infectious agent despite poor transmissibility to humans (52,53).
Acknowledgments
We thank Doctor Juan Sebastian Bravo, Infectious Disease Division at Military Hospital, Bogotá Colombia, Universidad Militar Nueva Granada and Fundación Universitaria Juan N. Corpas, Bogotá, Colombia. Finally we thank Dr. Nélida Forero Cubides for her helpful review of English manuscript.
Disclosure
The complete manuscript was prepared and reviewed for all authors. None of the writers have conflict of interest.
Referencias
1. World Health Organization. Avian influenza in humans http://www.who.int/influenza/human_animal_interface/avian_influenza/en/ Consultado el 23 de enero de 2013. [ Links ]
2. Kawaoka, Y. Flu transmission work is urgent. Nature. 2012. 48(5):1-2 [ Links ]
3. Center For Disease Control And Prevention. Avian Influenza: Current H5N1 Situation. Disponible en: http://www.cdc.gov/flu/avian/outbreaks/current.htm Consultado el 23 de enero de 2013. [ Links ]
4. Subbarao K., Klimov, A., Katz, J., Regnery, H., Hall, H., Bender, C., Huang, J., Hemphill, M., Rowe, T., Shaw, M., Xu, X., Fukuda, K., Cox, N. Characterization of an Avian Influenza A (H5N1) Virus Isolated from a Child with a Fatal Respiratory Illness. Science. 1998. 279(5349):393-396. [ Links ]
5. Vega, R., Reyes, G. El virus de la influenza. Rev. neumología y cirugía de tórax. 2007. 66(1):12-S14. [ Links ]
6. Herrero, L. El virus influenza y la gripe aviar. Rev. CIET. 2007. 50(1):13-19 [ Links ]
7. Godoy, P. Pandemia de gripe aviar: un nuevo desafío para la salud pública. Gac Sanit. 2006. 20(1):4-8. [ Links ]
8. Campins, M., Diez, J. Gripe aviar: ¿para cuándo la vacuna?. Med Clínica. 2006. 127(3):93-95. [ Links ]
9. Imai, H., Shinya, K., Takano, R., Kiso, M., Muramoto, Y., Sakabe, S. The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets. PLoS Pathog. 2010. 6(9):1-13 [ Links ]
10. Thomas Pg, Hertz T. Constrained evolution drives limited influenza diversity. BMC Biology. 2012. 10:43-46 [ Links ]
11. Garrett, L. ¿La próxima pandemia? Rev. Salud pública de México. 2006. 48(3):268-278 [ Links ]
12. Ibiapina C, Araújo G, Coutinho A. Avian influenza A (H5N1) - the bird flu. J Bras Pneumol. 2005. 31(5):436-44 [ Links ]
13. Ramos, C. La influenza, una oportunidad para la prevención y el control. Rev. Salud pública de México. 2005. 47(6):394-395 [ Links ]
14. Osterholma, M., Kelleya, N. Mammalian-Transmissible H5N1 Influenza: Facts and Perspective. mBio. 2012. 3(2):1-4 [ Links ]
15. Van Riel, D., Munster, V., Wit, E., Rimmelzwaan, G., Fouchier, R., Osterhaus, D., Kuiken, T. H5N1 Virus Attachment to Lower Respiratory Tract. Science. 2006. 312(5772):399. [ Links ]
16. Ungchusak, K., Auewarakul, P., Kitphati, R., Dowell, S., Auwanit, W., Puthavathana, P., Uiprasertkul, M., Boonnak, K., Pittayawonganon, C., Cox, N., Zaki, S., Thawatsupha, P., Chittaganpitch, M., Khontong, R., Simmerman, J., Chunsutthiwat, S. Probable Person-to-Person Transmission. The New England Journal of Medicine. 2005. 352(4):333-340. [ Links ]
17. Imperiale, M. Biosafety Considerations of Mammalian-Transmissible H5N1 Influenza. mBio. 2012. 3(2):3-5 [ Links ]
18. Di Pasquale, A., Garcon, N., Hanon, E. Cómo abordar la amenaza de la pandemia de gripe: la experiencia con la vacuna H5N1 adyuvada con AS03. Rev. Adm Sanit. 2009. 7(3):459-476. [ Links ]
19. Govorkova, E., Ilyushina, N., Marathe, B., Mcclaren, J., Webster, R. Competitive Fitness of Oseltamivir-Sensitive and -Resistant Highly Pathogenic H5N1 Influenza Viruses in a Ferret Model. Journal of virology. 2010. 84(16): 8042-8050 [ Links ]
20. Ortiz, J., Katz, M., Mahmoud, M., Ahmed, S., Bawa, S., Farnon, E., Sarki , M., Nasidi, A., Ado, M., Yahaya, A., Joannis, T. Akpan, R., Vertefeuille, J., Achenbach, J., Breiman, R., Katz, J., Uyeki, T., Wali, S. Lack of Evidence of Avian-to-Human Transmission of Avian Influenza A (H5N1) Virus among Poultry Workers, Kano, Nigeria, 2006. The Journal of Infectious Diseases. 2007. 196:1685-1689. [ Links ]
21. Reina, J., Ortiz, R., Lejarazu, R. Mecanismos de patogenicidad y adaptabilidad humana de las cepas gripales aviarias A (H5N1). Rev Esp Quimioterap. 2005. 18(4):273-280. [ Links ]
22. Cunha, C., Araújo, G., Coutinho, A. Avian influenza A (H5N1) - the bird flu. J Bras Pneumol. 2005. 31(5):436-44 [ Links ]
23. Uhart M, Karesh W, Smith K. Lecciones aprendidas de la Influenza aviar. Hornero. 2008. 23(2):61-66 [ Links ]
24. Imai, M., Watanabe, T., Hatta, M., Das, S., Ozawa, M., Shinya, K., Xhong, G. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/ H1N1 virus in ferrets. Nature. 2012. 48(1):1-9 [ Links ]
25. Yong, E. Mutant-flu paper published Controversial study shows how dangerous forms of avian influenza could evolve in the wild. Nature. 2012. 48(5):13-14 [ Links ]
26. Linzitto, O., Espinoza, C., Rodríguez, C., Pecoraro, M. Reseña sobre vigilancia y prevención de la influenza aviar y rol zoonótico. Acta Bioquim Clin Lat. 2005. 39 (4): 485-492. [ Links ]
27. Chan, R., Chan, M., Wong, A., Karamanska, R., Dell, A., Haslam, S., Sihoe, A., Chui, W., Baltzer, G., Li, Q., Malik, J., Nicholls J. DAS181 Inhibits H5N1 Influenza Virus Infection of Human Lung Tissues. Journal of virology. 2009. 59(9):3935-3941 [ Links ]
28. Torre, M., Ocampo, M., Lugo, C., Navarro, N. Características clínicas y epidemiológicas de la infección por el virus de la influenza a H1N1 en el Hospital General de San Cristóbal de las Casas. Rev. CENIC. 2010. 41: 1-10. [ Links ]
29. Yu, M., Zhang, CH., Yang, Y., Yang, Z., Zhao, L., Xu, L., et al. The interaction between the PARP10 protein and the NS1 protein of H5N1 AIV and its effect on virus replication. Virology Journal. 2011. 8:546-855 [ Links ]
30. Li, C., Bankhead, A., Eisfeld. A., Hatta, Y., Jeng, S., Chang, J., Aicher, L., Proll, S., Ellis, A., Law, L., Waters, K., Neumann, G., Katze, M. Host Regulatory Network Response to Infection with Highly Pathogenic H5N1 Avian Influenza Virus. Journal of virology. 2011. 85(21): 10955-10967 [ Links ]
31. Martin J., Loeches I., Rello J., Antón A., Almansa R., Xu L. Host adaptive immunity deficiency in severe pandemic influenza. Critical Care. 2010. 14(R167):1-12 [ Links ]
32. Ramos, I., Bernal, D., Durham, N., Belicha, A., Lowen, A., Steel, J., Fernandez, A. Effects of Receptor Binding Specificity of Avian Influenza Virus on the Human Innate Immune Response. Journal of virology. 2011. 85(9):4421-4431 [ Links ]
33. Almansa, R., Ortiz, R., Largo, E., Iglesias, V., Golvano, E., Bermejo-Martin, J. H5 influenza haemagglutinin and cytokine profiles in cultured PBMCs from adults and children. Inmunologia. 2011. 30:79-84 [ Links ]
34. Yu, W., Chan, R., Wang, J., Travanty, E., Nicholls, J., Malik, J., Mason, R., Chan M. Viral Replication and Innate Host Responses in Primary Human Alveolar Epithelial Cells and Alveolar Macrophages Infected with Influenza H5N1 and H1N1 Viruses. Journal of virology. 2011. 85(14): 6844-6855 [ Links ]
35. Vong, S., Sowath, L., Van Kerkhove, M., Achenbach, J., Holl, D., Buchy, P., Sorn, S., Seng, H., Uyeki, T., Sok, T., Katz, J. Risk Factors Associated with Subclinical Human Infection with Avian Influenza A (H5N1)-Cambodia 2006. The Journal of Infectious Diseases. 2009. 199(1):1744-752. [ Links ]
36. To, K., Chan, P., Chan, K., Lee, W., Lam, W., Wong, K., Tang, N., et al. Pathology of Fatal Human Infection Associated With Avian Influenza A H5N1 Virus. Journal of Medical Virology. 2001. 63:242-246 [ Links ]
37. Fica, A., Cifuentes, M., Ajenjo, C., Delpiano, L., Febre, N., Medina, W. Parada, Y. Precauciones en la atención de pacientes hospitalizados por influenza aviar H5N1. Rev Chil Infect. 2006. 23(4):290-296. [ Links ]
38. Khurana, S., Sasono, P., Fox, A., Van, N., Mai, L., Thai, P., Tran, N., Thanh, N., Horby, P., Golding, H. H5N1-SeroDetect EIA and Rapid Test: a Novel Differential Diagnostic Assay for Serodiagnosis of H5N1 Infections and Surveillance. Journal of virology. 2011. 85(23):12455-12463 [ Links ]
39. Montalvo, M., Resendiz, M., Santos, G., Vallejo, V., Reyes, J., Hernandez, J. Estandarización de un método de detección molecular del virus influenza (H5N1) de alta patogenicidad. Acta Bioquím Clin Lat. 2009. 43(1):49-52. [ Links ]
40. Oehadian A., Jusuf H., Pranggono E., Parwati I., Setiabudi D. Hematologic Manifestation of Avian Influenza Patients in Hasan Sadikin Hospital. Acta Med Indones-Indones J Intern Med. 2009. 41(3): 126-129 [ Links ]
41. De Andrade, C., Ibiapina, C., Champs, N., Castro, A., Mendoga, I. Avian influenza: the threat of the 21st century. J Brass Pneumol. 2009. 35(5):470-479. [ Links ]
42. Calvo, M. Vacuna para virus influenza H5N1. Rev Chil Infect. 2008. 25(4):310-312 [ Links ]
43. Bodewes, R., Kreijtz, J., Amerongen, G., Geelhoed, M., Verburgh, R., Heldens, J., Bedwell, J., Brand, J., Kuiken, T., Baalen, C., Fouchier, A., Osterhaus, D., Rimmelzwaan G. A Single Immunization with CoVaccine HT-Adjuvanted H5N1 Influenza Virus Vaccine Induces Protective Cellular and Humoral Immune Responses in Ferrets. Journal of virology. 2010. 84(16): 7943-7952 [ Links ]
44. US Department Of Health And Human Services. Press Statement on the NSABB Review of H5N1 Research. For Immediate Release. Tuesday, December 20, 2011. Disponible en: http://www.nih.gov/news/health/dec2011/od-20.htm. Consultado el 23 de enero de 2013. [ Links ]
45. Macchiavello, M. Teoría de la escalera térmica. Rev Chil Infect. 2009. 26(4):376-377 [ Links ]
46. Fouchier R., García A., Kawaoka Y., Barclay W., Bouvier N., Brown I., et al. Pause on Avian Flu Transmission Research. Science. 2012. 355:400-401. [ Links ]
47. Garcia, A. Working Safely with H5N1 Viruses. MBio. 2012. 3(2):1-3 [ Links ]
48. Enserink M. Ron Fouchier: In the Eye of the Storm. Science. 27th January 2012; 355:388-389. Disponible en: http://news.sciencemag.org/scienceinsider/2012/01/flu-researcher-ron-fouchier-its.html. Consultado el 23 de enero de 2013. [ Links ]
49. Perera, C., Díaz, H., Pérez, L. Actualización y perspectivas en el diagnóstico del virus de la influenza aviar. Rev. Salud Anim. 2011. 33(1):1-7. [ Links ]
50. Bresee, J., Hayden, F. Epidemic Influenza - Responding to the Expected but Unpredictable. N Engl J Med. 2013. 368:589-592 [ Links ]
51. Vajo, Z., Wood, J., Kosa, L., Szilvasy, I., Paragh, G., Pauliny, Z., Bartha, K., Visontay, I., Kis, A., Jankovics I. A Single-Dose Influenza A (H5N1) Vaccine Safe and Immunogenic in Adult and Elderly Patients: an Approach to Pandemic Vaccine Development. Journal of virology. 2010. 84(3):1237-1242 [ Links ]
52. Kolter, R.; Berns, K., Atlas, R., Ostroff, S. Docket Number: CDC-2012-0010. Disponible en: http://www.asm.org/index.php/programs2/public-policy/137-policy/documents/statements-andtestimony/90970-12-14-12-h5n1. Consultado el 23 de enero de 2013. [ Links ]
53. Baden L. For an Influenza Vaccine, Are Two Bs Better Than One?. N Engl J Med. 2013. 369(26):2547-2549 [ Links ]