Introduction
Since the isolation of human immunodeficiency virus type 1 (HIV-1) in 1983 1, fundamental advances have been made in understanding its biology, leading to the development of antiretroviral drugs that suppress virus replication. The study of viral components of proteins and their functions serves as a fundamental basis for developing new anti-HIV strategies. One of these widely studied components of proteins is its envelope glycoprotein (ENV), which is vital during the infectious process 2. Studies on this protein have focused mainly on the gpl20 subunit and the gp41 subunit ectodomain. On the other hand, the endodomain or Cterminal tail has received relatively little attention and is considered one of the least studied parts of the virus 3. However, the body of evidence currently reveals that CTT plays an essential role in the replicative cycle of HIV-1, which could be a vital determinant of the infectious process and a potential pharmacological target 4. The presence of a series of motifs that interact with cellular proteins linked to vesicular trafficking, death, and cellular activation has been observed 5,6. The compilation, synthesis, and analysis of all the evidence documented to date related to the functionality of these signal sequences are essential for raising new research questions in the area. The following review addresses the advances of more than three decades of studies on this domain, explicitly analyzing the identified motifs with which proteins interact, their roles in the biology of the virus, and their possible implications in the development of the disease.
General aspects
The extension of the CTT of most lentiviruses is notably more prolonged than that of other retroviruses, so it has been suggested that this feature plays an essential functional role in the lentiviral replication cycle 7,8. The roles of the CTT in the biology of HIV-1 have been demonstrated by a series of experimental studies about its influence on processes such as viral incorporation of the glycoprotein, immunogenicity, maturation, infectivity, and fusogenicity 7. The research interest in this region began with observations of the immunogenic properties of a sequence called the ‘Kennedy epitope,’ followed by observation of the viroporin activity of three amphipathic alpha-helices called lentiviral lithic peptides (LLPS), and finally by mutational mutagenesis studies that proved the importance of this domain in the assembly of new virions 9. Specifically, through the latter, a series of sequences and motifs that are important in the replicative cycle and involved in viral pathogenicity have been found (Table 1).
Table 1. Functionally identified motifs in the cytoplasmic domain of Env
| Motif | Position of the residues in Env | Function | References |
|---|---|---|---|
| YxxΦ* | 712-715 | Env intracellular trafficking and endocytosis. Polarized budding | 10-14 |
| YHRL | 768-771 | NF-kB activation | 15 |
| YW for Dileucine motifs | 795-796 | Env recycling | 16 |
| 802-803 | Env recycling | 17 | |
| 855-856 | Env endocytosis | 18 | |
| is1 and is2 | 749-760 and 761-783, respectively | Env recycling | 19 |
| Calmodulin binding site | 828-854 | Apoptosis | 20 |
(*) X is any amino acid, and Φ is a hydrophobic amino acid.
Source: Own elaboration
ENV traffic and incorporation
ENV is transported to the cell surface during the assembly process, where an interaction with the p17 domain (matrix) of the Gag protein allows its incorporation into the viral particle. This interaction occurs specifically between the ENV CTT region and an amino-terminal sequence of the matrix and represents one of the critical aspects of viral assembly. The remnant of the ENV protein that does not interact with Gag is then endocytosed to endocytic compartments from where it is recycled to the plasma membrane. A tyrosine-based internalization signal has been determined in the cytoplasmic domain of gp41, analogous to those found in some membrane proteins. It has been identified as responsible for the observed endocytosis of the envelope protein from the cell surface. The first to demonstrate the above were Rowell et al., who tried to explain why MHC class II proteins processed ENV, an endogenous protein 21. The authors found that this phenomenon was because this protein was endocytosed and processed as an exogenous agent. The endocytosis mechanism would be achieved through the interaction of the tyrosine-based motif YXXΦ (where X is any amino acid and Φ is a hydrophobic amino acid) with adaptin µ2, a member of the ap2 adapter protein complex associated with the plasma membrane 22,23.
It has been well established that the AP2 complex recruits the ENV in clathrin-lined wells for its internalization. The importance for the virus of preserving this endocytosis signal could be to impair the recognition of infected cells by the immune system by reducing the number of viral proteins exposed on the cell surface. In the presence of the Pr55gag precursor, the ENV internalization rate is particularly slow. An interaction between the p17 domain of the Gag precursor and ENV during the viral assembly process would prevent ENV interaction with the adaptins, thus allowing its incorporation into the virion 24.
Y712 replacement with a cysteine or phenylalanine residue has been observed to increase the ENV expression levels on the surface 25. Berlioz-Torrent et al. studied the role and effects of mutations on this YXXΦ motif proximal to the membrane on the distribution of envelope proteins of HIV-1, simian immunodeficiency virus (SIV), and human T-cell lymphotropic virus type 1 (HTLV-1). In this study, the researchers found that 712YXXΦ determined the expression levels of the different proteins on the cell surface; changes in the tyrosine-based motif resulted in a redistribution of the envelope protein. Particularly in HIV-1 and SIV, it was observed that the effects were less drastic compared to HTLV-1, so the researchers suggested the existence of another signal sequence determining the trafficking of their envelope proteins 26. This signal was later identified by Wiss et al., where a dileucine motif at the C-terminal end of CTT (L855-L856) interacts with the AP-1 complex and cooperates with motif 712YXXΦ to regulate ENV trafficking 27. Byland et al. demonstrated that, besides interacting with the AP-1 complex for env distribution, the dileucine motif at the C-terminal end of CTT also participates as an endocytosis signal. In this study, the authors showed that the 855LL sequence interacted with the AP-2 complex during the endocytic process 18.
To date, the evidence clarifies that this motif plays a modulatory role in ENV expression on the cell surface, which explains its conservation in primate lentiviruses. The modulating function of the envelope protein expression would enable a better dynamic of the infectious viral particle assembly on different sites of the infected cell. Indeed, it has been shown that viral assembly in macrophages, unlike T lymphocytes, also takes place in internal membranes, so the existence of this motif would directly affect ENV interaction with Gag in sites other than the cell membrane. Concisely, this dileucine-based motif would be called upon to collaborate with the 712YXXΦ motif in maintaining low ENV expression levels on the cell surface and, therefore, becoming a mechanism for viral evasion.
Since the late 1980s and early 1990s, it has been observed that the ENV expression is polarized towards the basolateral membrane in epithelial cells 13,29-31. However, it was not until Lodge et al. in an assay with Mardin-Darby canine kidney cells (MDCK) that the determining signal of this polarization, motif 712YXXΦ, was found (Fig. 1) 28. Interestingly, this phenotype was also noted in the T Jurkat lymphocyte line when a polarized targeting of ENV dependent on this tyrosine motif that coincided with viral budding was observed 10. Lymphocyte polarization has been linked to the cell-cell transmission through the virological synapse, a more efficient propagation mechanism than the virus-ffee-cell process 32. In vitro assays with other retroviruses, such as HTLV-1 and murine leukemia virus (MLV), have also demonstrated the functional importance of this tyrosine-based signal for this viral transmission mechanism 33,34. The conservation of these tyrosine-based polarization signals can play a fundamental role in the efficient urogenital transmission of the virus. Although epithelial cells are not the final target for HIV, their infection is decisive in the context of sexual transmission, which is the most critical transmission mechanism for HIV. Infection mechanisms of intact genital epithelial cells have been proposed by infected mononuclear cells present in genital tract secretions through cell-cell contact, independent of CD4 35. The polarized viral exit of the infected lymphocytes would greatly favor this process. Once the epithelial cells are infected, the basolateral exit of the secreted virions would, in turn, allow the infection of the mononuclear cells, important target cells in the connective tissue located next 36.
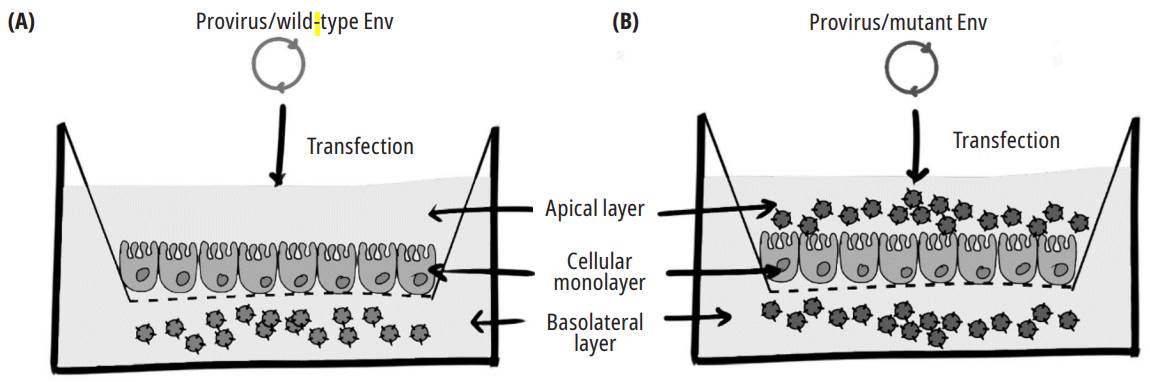
Source: Own elaboration
Fig. 1. An experiment that shows the effect of CTT motif Y712 on the polarized release of the virus. MDCK cells growing on semipermeable 0.45 pm-pore-diameter membrane were transfected with wild-type (A) and mutant (at position Y712 of ENV) viral genomes (B). Media in the upper and lower chambers were harvested 48 h after transfection, and the p24 antigen was detected using an enzyme-linked immunosorbent assay (ELISA) 28.
Another important finding is the involvement of the accessory protein Vpu in the infectivity of nascent virions. Studies carried out by Cervantes- Acosta et al. demonstrate a functional interaction of the accessory protein Vpu with the YXXΦ polarization motif 12,37. Although a close relationship was not detected between both proteins in their native state, it is surprising how Vpu acts to potentiate infectivity in the context of a 712YXXΦ mutant. The authors hypothesize that this accessory protein could serve as a facilitator of ENV targeting to specific membrane microdomains where assembly and release are more efficient 37. This phenotype was associated with the Vpu function linked to the increase in viral output and, mainly, an increase in the incorporation of the viral ENV in the virions.
The increase of ENV incorporation in the virion in such a context could be explained by the fact that, in increasing viral output, Vpu somehow increases or directs the assembly of viral structural components towards specific microdomains of the plasma membrane where the viral assembly process is more efficient by optimizing the quality of the virions. Viral infectivity determination tests using altered viruses in their Vpu ability to promote viral release were unable to increase the infectivity of a Y712 mutant. On the other hand, immunoprecipitation experiments showed an increased incorporation in the virion of the envelope protein that contained the mutant compared to the wild-type protein in normal Vpu 37.
ENV incorporation in virions seems to be a cell-specific process. Mutational studies with different cell lines show different behaviors in ENV trafficking 38. Some truncations in CTT do not seem to affect ENV incorporation in specific cells, such as 293T or HeLa cells. Due to this characteristic, these cell fines are called permissive cells. In contrast, cells in which the complete domain of CTT is strictly necessary for env incorporation are called nonpermissive, including most T lymphocyte fines, primary T cells, and monocyte-derived macrophages. Everything seems to indicate that certain regions of CTT, particularly the motifs that operate in ENV traffic, may or may not be essential depending on the cell fine 7. Qi et al. observed first that in the HeLa and H9 cell fines, ENV incorporation depended on the Rab14 protein and the FIP1C effector 39. Rab14 has been identified as a GTPase involved in vesicular trafficking. Rabl4 function consists of determining the targeting of the vesicle 40. This protein is bound by FIP1C, also known as Rab-coupfing protein (RCP), which functions as an adapter molecule 41. The complex is then responsible for recycling membrane proteins from the endocytic compartments. In the proposed model for HIV, FIP1C directs ENV incorporation into the viral particle in a CTT-de- pendent manner. This ENV incorporation is possible through the recruitment of Rab14 by FIP1C. CTT dependence on ENV incorporation is given by the YW795 motif, which interacts with Rab14/FIP1C. The YW795 mediation in the process occurs in a cell-type-dependent manner 17. Mutations in F1P1C, at least in HeLa cells, cause ENV to be trapped in endosomal recycling compartments (ERC), thus generating non-infectious virions. According to this proposal, ENV is targeted to ERCS before targeting the viral assembly site (Fig. 2) 16.
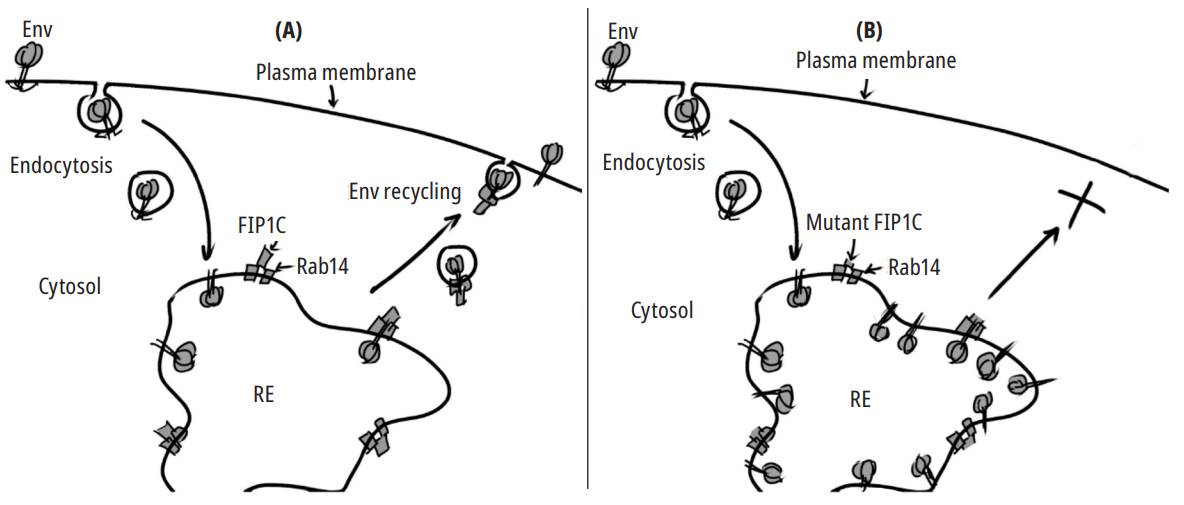
Source: Adapted from 16.
Fig. 2. Env recycling from the endocytic compartments. In a model based on HeLa cells, Env is recycled from endosomes to the plasma membrane (PM). This process is mediated by the GTPase Rab14 and its effector FIP1C (A). Mutations in the CTT domain or Rab14 inhibit transport to the MP and cause Env accumulation in recycling endosomes(REs) (B).
A more recent mutational study demonstrated the effect of two truncations in CTT on viral replication. A first CTT variant had a cut on the amino acid at position 716, just after the 712YXXØ motif, and a second on position 813, 18 residues downstream of the YW795 motif. The first caused a total inhibition of replication, curiously in both permissive and nonpermissive cells. The effect of the second variant was more diverse; in the 293T and MT-4 cell fines, ENV incorporation and infectivity in virions were maintained. However, it was defective in CEM-SS cells, a fine categorized as nonpermissive 42.
The ENV recycling process has also been directed towards the trans-Golgi network (TGN) as an alternative route. Two studies revealed that mutations made in the 802YW motif reduce ENV expression on the surface, affecting its incorporation in the new virions 43,44. One of those studies reported that recycling is mediated by the perilipin-3 protein, also known as TIP47. It recognizes the 802YW motif, just like a signal sequence in the cytoplasmic domain of mannose-6-phosphate receptors does for its retrograde transport from endosomes to the TGN 45.
Other Rab GTPases have been identified as mediators of ENV vesicular trafficking. Studies where the role of Rab9, a GTPase that interacts with TIP47, was examined showed that both the silencing of Rab9 and its effector, TIP47, dramatically inhibited viral replication, supporting previous findings 43,46. GTPase Rab9 is involved in the vesicular transport pathway, specifically in the targeting and fusion functions between the small transport vesicles and the different target organelles, acting as a promoter of the assembly of integral protein- membrane complex, the VSNARES (soluble N-eth- ylmaleimide-sensitive factor attachment protein receptor), located in the transport vesicle, with its corresponding pairs, the t-SNAREs, located in the target membrane 47. Rab9 facilitates transport from late endosomes to the TGN by coupling with its effector p40. Rab9 has also been reported to bind to the vesicle cargo selection protein TIP47, whose interaction with CTT was critical for ENV incorporation into mature virions. The silencing of Rab9 matched with the Gag protein localization in the endocytic compartments, which suggests that the defect in HIV replication may result from an inhibition of endocytic protein trafficking out of late endosomes.
Another GTPase involved in vesicular transport, Rab11 A, also appears to be essential for ENV trafficking, although its role was unclear 46. While the 802YW motif is a determinant for ENV incorporation, the role of the TIP47 effector is not apparent. The participation of this protein was questioned in a recent study, where its inhibition did not affect ENV incorporation, the efficiency of release, or the infectivity of virions 48.
An identified component that would be key for ENV recycling is the retromer, the protein complex that operates in the retrograde transport of cargo proteins from the endosomes to the Golgi 49. A study by Groppelli et al. showed that the retromer regulates ENV trafficking and is directly related to the infectivity of viral progeny. The inhibited formation of this complex disturbs ENV recycling from the endocytic compartments to the Golgi. In addition, they observed that the infected cells showed a higher ENV expression on the surface, which increased their incorporation in the nascent virions and resulted in greater infectivity 39. Now, specifically, the regions in CTT identified by Gropelli et al. that directly interacted with the retromer were called is1 (V747-S760) and is2 (1761-1783), which had previously been involved in ENV trafficking 50,51. The deletion of these two sequences resulted in the lost ability to bind to the components of the complex 19.
ENV incorporation into the viral particle responds to highly regulated vesicular transport events. It begins with exporting the protein to the plasma membrane using AP-1. Once on the surface, it can interact with the Gag ma domain to be incorporated into a new virion, or it can be endocytosed through AP-2. The endocytosed protein is recycled from the endocytic compartments directly towards the cell membrane or towards the trans-Golgi face thanks to the retromer (Fig. 3). It remains to be elucidated whether some proteins that interact with CTTS linked to trafficking, such as GTPases and effectors, do so in a cell-specific way or in a constitutive way.
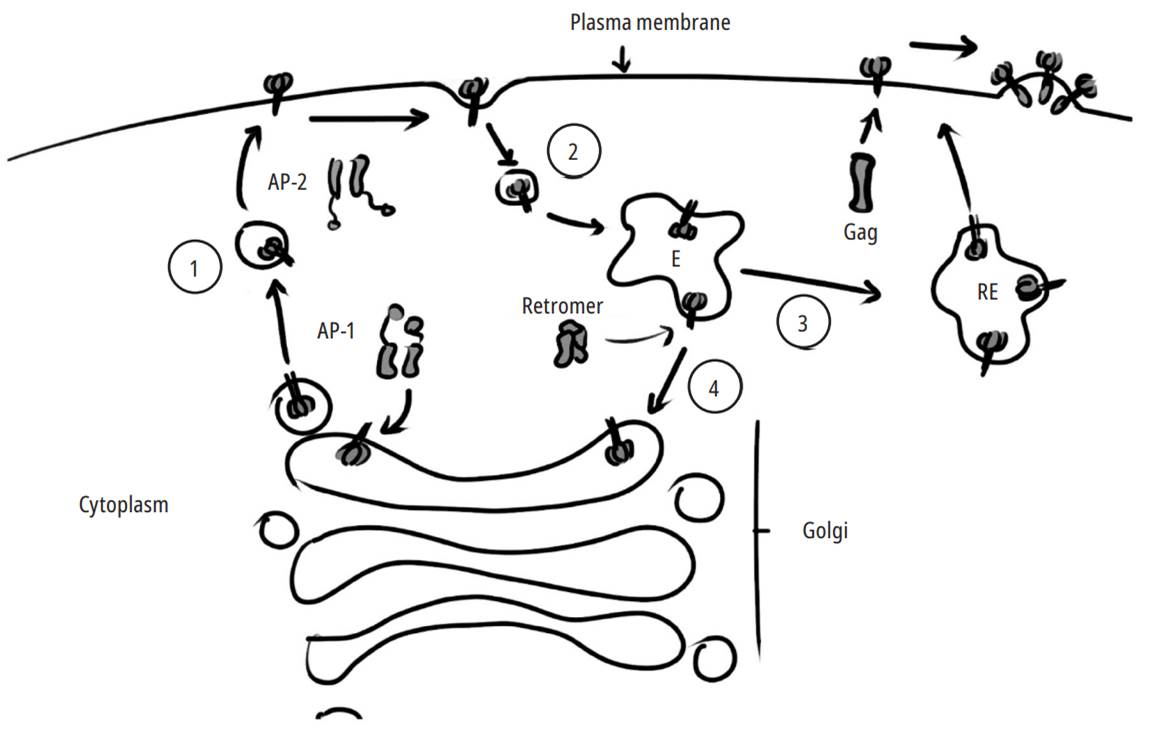
Source: Own elaboration
Fig. 3. Env trafficking, incorporation, and recycling. Env is transported from the trans-Golgi network to the basolateral membrane via AP-1 in polarized cells (1). Once on the surface, if it does not interact with the Gag MA domain, Env undergoes rapid internalization mediated byAP-2 (2). Then, in the endocytic compartments (E), it can be recycled by two pathways: a fast pathway through the recycling endosomes (REs) to the plasma membrane (3) or a slow pathway from the early or late endosomes towards Golgi mediated by the retromer (4).
It has been suggested that the regulation of ENV expression by endocytosis seems to be a strategy for evading the immune system. The surface presence of this glycoprotein in the infected cell makes it a target of antibodies or a mechanism of cytotoxicity, so its minimal expression would provide a degree of protection against a cellular or humoral response 19,24,52. It uses the vesicular transport machinery to maintain basal levels of the protein in the membrane, which allows it to incorporate sufficient ENV complexes in the nascent virions while remaining hidden from an immune response. The importance of envelope protein trafficking modulating motifs, as evidenced, suggests that it is a crucial factor in the replicative efficiency of some viruses, at least in homologous viruses 53,54. In the study by Fultz et al. with SIV, macaques were inoculated with a virus variant in the Y721 endocytosis/polarization motif. The experiment attenuated pathogenicity, maintaining low or undetectable viral loads in the plasma and normal percentages of CD4+ T cells 54.
Cell activation
HIV-1 mainly infects T lymphocytes, which must be activated to result in efficient viral replication. It has been shown that CTT can induce cellular activation by nuclear factor kαppα light chain enhancer of activated B cells (NF-KB) 15. NFKB is a family of transcription factors that regulate various cellular processes, such as immunity, proliferation, differentiation, and survival 55,56. NF-KB activation begins with the interaction of CTT with TGF-β-activated kinase 1 (TAK1), a member of the mitogen-activated kinase family, CTT physically interacts with TAK1 through the tyrosine-based motif 768Yxxφ15. Since the late 1980s, it has been known that HIV-1 possesses NF-KB binding sites in its LTR, and these sites function as enhancers of proviral expression 57, such that CTT-mediated activation of NF-KB would function as a positive feedback mechanism that stimulates viral production. Postler et al., in their study, tested this with HIV-1 NL4-3 mutants, which lacked the activation signal of TAK1. In the trial, they showed that the mutant viruses did not suffer from deficiencies in their replication in active T lymphocytes, while they were unable to replicate under conditions of suboptimal cellular activation 15.
Cell death
Since HIV-1 was isolated, it has been suggested that the cytoplasmic domain of gp41 is responsible, at least in part, for the cytopathic effects observed in cell cultures 58,59. On the other hand, it has been shown that CTT interacts with calmodulin (CaM), a protein that regulates apoptosis 60. CaM is responsible for controlling intracellular Ca2+ concentrations and is involved in multiple cellular processes by modulating the activity of many proteins 61. The binding of CTT with CaM inhibits its activity and consequently induces an increase in the cytosolic Ca2+ concentrations, which leads to apoptosis 52. An initial study determined that the region involved in CaM binding lies in the ENV cytoplasmic domain. For the first time, it is suggested that this interaction induces a cytotoxic effect due to the inhibition of CaM functions 62. In the same year, LLP-1 was identified as the peptide determining this binding 63. Although subsequent studies by Sasaki et al. supported the CaM/ CTT association, it was not until 2006 when Micoli et al., through coimmunoprecipitation studies, determined the presence of critical residues in CTT for binding to CaM, whose mutation reduced Fasmediated apoptosis 20,64. This interaction would seem inconsistent with viral production since the induction of apoptosis of the infected cell would prevent efficient replication of the virus. However, it is hypothesized that, considering the low conservation of these CaM-binding residues, it would be expected that the most pathogenic CTTS would be less polymorphic in this region in the late stages of the disease 52,60
Circulating viruses
The studies show that the gp41 CTT contributes to a series of essential functions for viral replication and disease development. Despite this, few studies have been conducted to evaluate the conservation of this domain in circulating viruses. Lambelé et al. detected HIV-1 variants in four patients with natural polymorphisms in the motifs Y712 and 802YW. The CTTS derived from these patients were subsequently studied in vitro with confocal microscopy to demonstrate the effect on intracellular trafficking and the distribution of ENV, results that coincide with the mutational studies previously done with laboratory strains such as those of Berlioz-Torrent et al.44. Moreover, a patient that a variant of the virus, containing a deletion of 20 residues in its CTT that affected several motifs, maintained its ability to replicate. This ability was given by a substitution in the matrix protein (MA), which compensated for the expected negative effect of truncation 65. A more recent study compared the infectivity of wild-type viruses belonging to subtypes C and B. The authors show that the low replicative capacity of the former, already noted under in vitro conditions in other studies, is partly due to the differences in CTT 3. Most likely, researchers infer that the attenuated infectivity of this ENV region reduces the immunogenic and cytopathic effects and would benefit the virus in establishing persistent infection. Therefore, all the data indicate that polymorphisms in this domain are critical and direct determinants of HIV-1 pathogenicity 3,66,67.
Conclusions
The presence of signal sequences in the C-terminal domain of the HIV gp41 protein that interacts with some cellular and viral factors are critical in the replicative cycle and, generally, in the virus biology. Strict regulation in ENV incorporation into nascent virions by these signals is decisive in aspects related to the infection cytopathogenicity and the viral progeny infectivity; a higher ENV viral uptake has been associated with more infectious virions. ENV polarization towards specific membrane domains, regulated by these signals, seems to favor the efficiency of cell-cell transmission over transmission by free viruses. Although evidence is limited, the fact that the virus exits only through certain particular regions, such as pseudopods in T cells, could be considered as taking advantage of a kind of synapse as an infection mechanism towards other cells. Additionally, the concentration of viral assembly in one pole of the infected lymphocytes and the basolateral gemmation in polarized epithelial cells could play a determining role in sexual transmission by serving as a mechanism for crossing the intact genital epithelium through independent CD4 infection and later infection of mononuclear cells in connective tissue. The C-terminal domain of the HIV gp41 protein could regulate the viral transcriptional rate and, therefore, the infection development; ENV appears to function as positive feedback of viral transcription by activating the NF-kB canonical pathway. An increase in ENV production results in an increase in genome transcription and, therefore, an increase in viral titers.
In short, the implication in the immune response evasion by ENV is an aspect that has been much evidenced: ENV’S continuous intracellular circulation, transport towards the cell membrane, endocytosis, and recycling allow the virus to maintain the minimum necessary levels of ENV exposed on the cell surface, making it difficult for the immune system to recognize the infected cell. The series of studies carried out so far that shows us the importance of HIV CTT in many aspects of viral pathogenesis opens a door to scrutinizing the existence of similar motifs in other enveloped viruses that interact with cellular and viral proteins that can influence the biology and quality of the produced viruses. It would be important to know how these interactions depend on the intracellular location of the host proteins and the viral assembly site. These studies would be of singular scope considering that the symptom development of a productive viral infection depends to a great extent on the organs colonized by the virus and the polarization of the viral output from the infected organs. Another aspect to consider would be whether the different signals potentially present in the studied viruses would be interpreted differently in each cell. The influence of the viral assembly site on the differential incorporation of host proteins into the virus and its consequences for the quality of the produced virions would be a question to be answered about other potential functions of the viral envelope intracytoplasmic region.
It is crucial to project more research in the future to understand the mechanism of the interactions between the domain and cellular and viral factors. It is necessary to perform studies on the CTT sequences of circulating viruses and the various cellular proteins that interact with the domain. Expanding the knowledge on the epidemiological behavior of this region of the glycoprotein, which is under selective pressure, and determining the level of conservation of the motifs would ultimately provide the possibility of using this region as a potential target for treating the infection. Similarly, polymorphisms in cellular proteins interacting with CTT may likely be a determining factor in tolerance to the development of the disease.














