DURING ADOLESCENCE, animal behavior is modulated by important changes in morphology and brain functioning (Doremus-Fitzwater et al., 2010; Sharma et al., 2013). For instance, brain development of the prefrontal cortex and the limbic system affects decision making and risk-taking behaviors (Goriounova & Mansvelder, 2012; McCutcheon & Marinelli, 2009). Furthermore, it has been reported that adolescent rats tend to explore novel environments and perform risk-related behaviors more frequently than adult rats (Bishnoi et al., 2020; Stansfield & Kirstein, 2006). Similarly, maturation of neuroendocrine systems as the hypothalamus-pituitary-gonadal axis (HPG axis) and the hypothalamus-pituitary-adrenal axis (HPA axis) contributes to physical and emotional changes, including increased sensitivity to environmental demands and potential use of addictive drugs (Eiland & Romeo, 2013).
Different studies have found that exposure to nicotine stimulates dopaminergic neurons in the mesolimbic circuit, this effect is associated with the rewarding and addictive properties of drugs of abuse (Yuan et al., 2015). Sustained activation of this circuit by prolonged drug exposure promotes locomotor sensitization in experimental animals (Bernardi & Spanagel, 2014). Locomotor sensitization has been observed with the administration of different types of drugs and has been used as an experimental paradigm to study common neuroplastic changes underlying behavioral adaptation to drugs of abuse. (Camarini & Pautassi, 2016; Levine et al., 2011; Volkow, 2011). However, further research is needed to address remaining questions on developmental factors. For instance, few studies have assessed nicotine-induced locomotor sensitization among different developmental stages, and those that are available report inconsistent results (DiFranza & Wellman, 2007; Falco & Bevins, 2015). Some studies reported higher sensitization responses for adolescent rats (Adermark et al., 2015; Faraday et al., 2001), whereas other studies reported higher sensitization responses for adults (Schochet et al., 2004; Zago et al., 2012). Such differential sensitivity responses might be related to the brain's developmental state at the beginning of drug exposure (Cao et al., 2010; Counotte et al., 2011).
In addition to locomotor stimulating effects of nicotine, other studies have evaluated emotional effects induced by nicotine with paradigms frequently employed to assess anxiety-like responses in animal models (Bishnoi et al., 2020; Counotte et al., 2011; Morud et al., 2018; Picciotto & Kenny, 2020). Since exploratory behavior of rodents in the open-field (OF) is determined by the intrinsic conflict between exploration of and aversion to open, bright areas, this instrument is a valuable tool to study modulation of behavioral inhibition and to measure emotional-locomotor interactions (Lamprea et al., 2008; Prut & Belzung, 2003). In particular, the evaluation of exploratory behavior in specific areas of the OF, like activity in the center zone, can be interpreted as an indicator of behavioral disinhibition or risk-related behaviors (Bishnoi et al., 2020; Meert, 1986). Moreover, Carola et al. (2002) reported correlations between the behavior in the OF center zone and the activity in the open arms of the elevated plus-maze (EPM), which suggests both instruments may be used to assess similar behavioral processes. Thus, given the opportunity to evaluate risk-related behaviors on the OF procedure, the present study assessed the emotional and locomotor effects of nicotine in adolescent and adult rats on the OF, with the general goal to advance our understanding of behavioral adaptations to nicotine administration in animals from different developmental stages.
Method
Subjects
Thirty-nine adolescent male Wistar rats on postnatal day (PD) 28 and thirty-nine adults on PD 63, were supplied by Instituto Nacional de Salud, Bogotá. Adolescents weighted 96 ± 2.5 g and adults 341 ± 3.3 g upon arrival to the laboratory. Animals were group-housed and maintained in a sound-attenuated room with controlled humidity (50 ±3%) and temperature (22 ±1°C), under a 12hour light-dark cycle, lights on at 7:00 h with ad libitum access to water and food. After arrival to the laboratory, subjects were acclimatized to the housing conditions for one week before the experimental procedure. During the last three days of this period, animals were handled for five minutes once a day before experimental procedures began. All procedures were conducted between 7:00 and 13:00 h. This study was conducted according to Colombian Law and international guidelines: us National Institutes of Health guide for the care and use of laboratory animals. Experimental procedures were approved by the ethical committee at Fundación Universitaria Konrad Lorenz (CICUAL-Konrad Lorenz).
Drug Treatment
Nicotine hydrogen tartrate (Glentham Life Sciences, GL9693) was dissolved in sterile saline (0.9% NaCl) at 0.14 mg/ml (free base) and the solution was titrated to a pH -7.4 with NaOH 0.1M. Half of the animals were injected (S.C.) daily for 21 days with nicotine, 0.14 mg/kg body weight (Nicotine group), and the other half were injected (S.C.) with sterile saline 1 ml/kg body weight (Vehicle Group).
Instruments
Locomotor activity tests were performed in an OF consisting of a squared black acrylic box 60 x 60 x 60 cm. The OF was uniformly illuminated from the ceiling, around 60 ± 2 lx. All the tests were videotaped and processed through ANY-maze behavioral tracking software to register horizontal activity. The OF was virtually divided in two zones for posterior analyzes: Center and Periphery. Center was a square in the middle of the field with an area of 20 x 20 cm and the Periphery was the rest of the field.
Procedure
On the first day of experimental procedures, all animals were submitted to a baseline locomotor activity test consisting of 5 minutes of free exploration of the OF. Immediately after, animals were randomly assigned to either Nicotine or Vehicle groups and received the first nicotine or saline injection. Following injection, animals rested for 10 minutes within the transporting cage, time required for brain nicotine levels to peak (Matta et al., 2007), and then were submitted to an acute-challenge locomotor activity test. From days 2 to 20, subjects were transported out of the vivarium for treatment administration, and immediately after were returned to their home cages. On day 21, animals were submitted to a long-term re-exposure locomotor activity test. Immediately after, animals received the last saline or nicotine injection. After this injection, animals rested for 10 minutes within the transporting cage, and then they were submitted to a chronic-challenge locomotor activity test. Housing, injections, and tests took place in different rooms and animals were transported in an opaque plastic cage between rooms. Treatments were applied from PD 35 to 55 for the adolescent group (PD 35 Group), and from PD 70 to 90 for the adult group (PD 70 Group).
Statistical Analysis
Data are presented as mean values relative to vehicle groups ± SEM. The significant level was set up to a=.05. Data were analyzed using SPSS v.22 software, performing Mixed ANOVA models. Two tailed Holm-Sidak's post hoc tests were used for additional comparisons. Total meters traveled were used as an indicator for the locomotor sensitization effects of nicotine. Meters traveled in the center zone and seconds spent in the center zone were used as indicators of the emotional effects of nicotine.
Results
Adolescent and Adult Rats are Equally Responsive to Nicotine-Induced Locomotor Sensitization
Chronic nicotine treatment induced locomotor sensitization in both adolescent and adult rats (Figure 2). Mixed ANOVA analysis showed no main effect for age, but there was a significant interaction between test and treatment (Test x Treatment: F(1/74)=23.45, p<.01, η 2 p=.24). Post hoc analysis showed no difference in the magnitude of sensitization between adolescent and adult rats (F(1/74)=I.60, p>.05, η 2 p =.02). There was an increase in distance traveled by nicotine-treated rats relative to vehicle-treated rats only during the chronic challenge test for both age groups (adolescent rats Acute: F(1/74)=.01, p>.05, η 2 p <.01; Chronic: F(1/74)=20.82, p<.01, η 2 p =.22; adult rats Acute: F(1/74)=1.14, p>.05, η 2 p=.02; Chronic: F(1/74)=11.14, p<.01, η 2 p=.13). In the same way, locomotor response to the chronic challenge was higher than the response to acute challenge (Chronic against Acute challenge test: F(3/72)=35.29, p<.01, η 2 p =0.60). Furthermore, it was not observed a sustained increase in locomotor activity as distance traveled by nicotine-treated rats was not different relative to vehicle-treated rats during the re-exposure test (Re-exposure: F(1/74)=2.80, p>.05, η 2 p =.04).
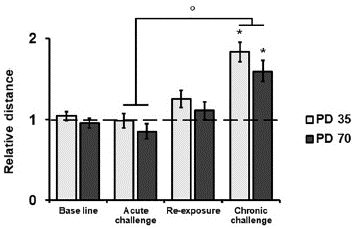
Figure 2 Relative locomotor activity of nicotine treated rats. Distance traveled is presented as a relative measure of mean distance travel by age paired vehicle-treated rats during every locomotor activity test. Dashed line represents the mean distance traveled by the vehicle group. * p<0.05 against vehicle group; ° p<.05 against acute challenge test for both ages.
Nicotine Disrupts Inhibition of Risk-Related Behavior in Adolescent but not in Adult Rats
General locomotor activity was analyzed focusing on distance traveled in the OF center zone. Nicotine increased the exploration of the center zone for adolescent but not for adult rats in both acute and chronic challenges (Figure 3). There was a significant increase in distance traveled in the center zone by nicotine-treated adolescents relative to vehicle-treated animals during acute challenge (Acute: F(1/74)=5.88, p<.05, η 2 p =.07; Chronic: F(1/74)=379, p>.05, η 2 p =.05). This effect was not observed in adult rats (Acute: F(1/74)=.07, p>.05, η 2 p =.01; Chronic: F(1/74)=.II, p>.05, η 2 p =.01). Furthermore, nicotine treated adolescents travelled a larger distance in center zone during both challenges compared to adults (Acute: F(1/74)=8.06, p<.05, η 2 p =.10; Chronic: F(1/74)=5.77, P<.05, η 2 p =.07). Moreover, there were no differences in distance traveled in the center between acute and chronic challenges for nicotine-treated animals (Adolescents: F(1/74)=71, p>.05, η 2 p =.01; Adults: F(1/74)=.O1, P>.05, η 2 p <.01) neither for vehicle-treated animals (Adolescents: F(1/74)=.01, p<.05, η 2 p =.01; Adults: F(1/74)=.77, p>.05, η 2 p =.01). Besides, there were no differences for vehicle-treated animal compared by age (Acute: F(1/74)=.00, p>.05, η 2 p =.00; Chronic: F(1/74)=.68, p>.05, η 2 p <.01) (Figure 3B).
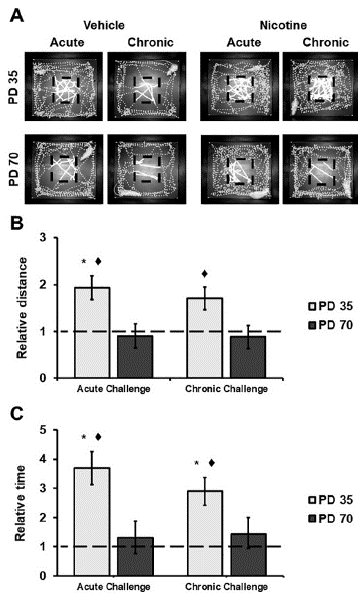
Figure 3 Exploration patterns in the open field. A. Black dashed lines represent the open field center zone. The distance traveled in this zone is highlighted. Representative tracks are presented by age, treatment, and challenge. B. Distance traveled in the center zone is presented as a relative measure of mean distance traveled in the zone by age paired vehicle-treated rats during each locomotor activity test. C. Time spent in the center zone is presented as a relative measure of mean time spent in the zone by age paired vehicle-treated rats during each loco-motor activity test. Dashed lines represent the mean distance traveled by the vehicle group. * p<.05 against vehicle group; °p<.05 against adults.
Similar effects were observed on time spent in the OF center zone. Nicotine increased time spent in the center for adolescent, but not for adult rats (Figure 3C). There was a significant increase in time spent in the center zone by nicotine-treated adolescents relative to vehicle-treated animals in both challenges (Acute: F(1/74) = 10.05> P<.05> η 2 p = .12; Chronic: F(1/74) = 6.79, p<.05, η 2 p =.08). This effect was not observed for adult rats (Acute: F(1/74)=.12, p>.05, η 2 p <.01; Chronic: F(1/74)=.34, p>.05, η 2 p =.01). Furthermore, nicotine treated adolescents spent more time in center zone during both challenges compared to adults (Acute: F(1/74)=8.91, p<.05, η 2 p =.11; Chronic: F(1/74)=4.58, p <.05, η 2 p =.06). Moreover, there were no differences in time spent in the center between acute and chronic challenges for nicotine-treated animals (Adolescents: F(1/74)=1.97, p >.05, η 2 p =.03; Adults: F(1/74)=.05, p>.05, η 2 p <.01) neither for vehicle-treated animals (Adolescents: F(1/74)=.04, p >.05, η 2 p >.01; Adults: F(1/74)=.15, p >.05, η 2 p >0.01). Besides, there were no differences for vehicle-treated animal compared by age (Acute F(1/74) = .00, P>.05, η 2 p =.00; Chronic: F(1/74)=.04,p>.05, η 2 p =.00).
Discussion
Chronic nicotine administration produced similar levels of locomotor sensitization on the evaluated age groups. In addition, acute and chronic nicotine challenges enhanced risk-related behaviors for adolescent rats in the OF. Consistent with our first result, Adermark et al. (2015) showed that adolescent and adult male Wistar rats developed a comparable nicotine-induced locomotor sensitization after a three-week treatment with the drug, under a similar procedure to the currently reported. However, previous research showed that adolescent male rats are more sensitive than adult male rats to nicotine stimulatory effects on locomotor activity (Faraday et al., 2001). An important detail on Faraday et al. work is that the drug was administered via osmotic minipumps. Less sensitive responses to nicotine in adults might be related to this continuous pharmacological administration, as continuous dosing with nicotine blocks the expression of sensitization in rats (Benwell et al., 1995). It is possible that this blockage only occurs in adult rats, when considering age differences in the metabolization of nicotine (Craig et al., 2014), nicotinic acetylcholine receptors density and sensitivity (Cao et al., 2010), and dopamine release in response to stimulant drugs (Thorpe et al., 2020; Yuan et al., 2015).
Sensitization to nicotine has been associated to changes in activity on extended limbic structures that could be related to processes of drug dependence (Li et al., 2008; Volkow, 2011). Some nicotine sensitization studies have reported increased dopamine and noradrenaline release into the prefrontal cortex (Fredrickson et al., 2003; Nisell et al., 1996) and nucleus accumbens after daily treatments (Cadoni & Di Chiara, 2000). Moreover, molecular studies have shown that Di dopamine receptors play a key role for locomotor sensitization, as sensitized animals showed enhanced responses to Di dopamine agonists (Kalivas, 1995), and Di dopamine antagonists blocked locomotor sensitization expression (Goutier et al., 2015). Otherwise, some studies have shown that psychostimulant drugs increase levels of impulsive response and disinhibition in humans and laboratory animals (Kolokotroni et al., 2011). Decreased behavioral inhibition observed in animals under exposure to drugs of abuse is related to neural changes similar to those observed in sensitized animals. For instance, Di dopamine antagonist administration can reduce premature responding (van Gaalen et al., 2006), and nicotine exposure stimulates dopamine release into the nucleus accumbens, which in turns elicit impulsive behavior (Cadoni & Di Chiara, 2000; Counotte et al., 2009; Ohmura et al., 2012).
In the present study, we found that acute nicotine and chronic nicotine challenges increased OF center exploration for adolescent but not for adult rats. Additionally, exploration of the center zone in absence of nicotine did not increase during the re-exposure test (data not shown). Therefore, the modulation of risk-related behaviors occurred only under the acute effect of nicotine. As mentioned before, Carola et al. (2002) proposed that center zone avoidance in the OF is comparable to avoidance of open arms in the EPM. Thus, a similar effect of acute administration of nicotine could be expected in both instruments. In this context, our results are consistent with those reported by Elliott et al. (2004), describing increased EPM open arms exploration by adolescent rats acutely exposed to nicotine. Additionally, some authors have proposed that open arms avoidance in the EPM results from inhibition of exploration in potentially dangerous regions (Olausson et al., 2001). Following this rationale, increased OF center zone exploration observed in our study might reflect nicotine's capacity to acutely decrease the inhibition of risk-related behavior in adolescents, but not in adults.
Risk-related behaviors and impulsive actions have been proposed as closely related processes, since both could be associated to poorly conceived or prematurely expressed actions often related to inappropriate responses and poorly adjusted behavior (Ohmura et al., 2012). In the present study, increased exploration in the OF center zone for nicotine-treated adolescents could be understood as a failure to withhold the exploratory drive in a conflict situation. An enhanced exploratory drive could be related to a nicotine-induced acute disinhibition of risk-related behaviors characteristic of adolescents. The age-dependent effects described in our work are consistent with previous suggestions that nicotine exposure during adolescence might result in increased impulsive action during adulthood, altered maturation of limbic structures (Counotte et al., 2009; Counotte et al., 2011), and increased vulnerability to nicotine addiction (Adermark et al., 2015).
Finally, it is worth noting that the dose used in the present experiment is one of the lowest reported to produce locomotor sensitization and other behavioral effects (DiFranza & Wellman, 2007; Le Foll & Goldberg, 2005), since this effect has usually been reported with higher doses (Schochet et al., 2004). As we reported, daily administration of 0.14 mg/kg nicotine during a three-week period produced a nearly two-fold increase in nicotine-related locomotor response for both adult and adolescent rats. Differences between the low and high drug doses could be related to the procedures used to assess locomotor activity sensitization. For the present work, animals were exposed to four OF tests, five-minutes long each, whereas other studies exposed animals to higher number of trials and for periods longer than fifteen minutes (Gabriel et al., 2019; Morud et al., 2018). Longer exposure time to the OF may reduce the exploratory drive commonly observed for rodents in novel environments, which could explain the need to use higher doses on previous research. Furthermore, our experimental setup may have facilitated the expression of risk-related behaviors on the OF center zone due to preserved spontaneous locomotor behavior (Matta et al., 2007). Thus, the present work can have a translational value for future nicotine addiction research, as low nicotine doses could better resemble adolescents' low rate of tobacco consumption (Abreu-Villaça et al., 2003).
Together, our results suggest that chronic daily exposure to nicotine promotes the potentiation of its stimulant effects on locomotor activity for both age groups. In adolescents, acute nicotine also decreased capacity to inhibit risk-related behaviors, even after repeated exposure to the OF. These stimulant and emotional effects for adolescents may be associated with changes in morphology of limbic brain regions (Counotte et al., 2011), higher dopaminergic reactivity to nicotine (Yuan et al., 2015), and increased drive for risky decision-making (Casey & Jones, 2010)
This research was financed by grants from: Fundación Universitaria Konrad Lorenz; Colombian Science, Technology and Innovation Ministry (Minciencias #772/ 2018) and Universidad Nacional de Colombia (DIB 41776 and FCH 43519).