Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ciencia en Desarrollo
Print version ISSN 0121-7488
Ciencia en Desarrollo vol.5 no.2 Tunja July/Dec. 2014
Heterogeneous Catalytic Oxidation of R-(+)-Limonene on Jacobsen Type Catalysts
Oxidación catalítica heterogénea de R-(+)-limoneno sobre catalizadores tipo Jacobsen
J. A. Cubillos Loboa,*
L. A. Páez Guevaraa
G. P. Romanellib
a Grupo de Catálisis-UPTC. Facultad de Ciencias. Universidad Pedagógica y Tecnológica de Colombia (UPTC), Tunja, Boyacá.
* Autor de correspondencia: jairo.cubillos@uptc.edu.co
b Centro de investigación y Desarrollo en Ciencias Aplicadas "Dr. J.J. Ronco" (CINDECA), Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Argentina.
Recepción: 25-jul-14 Aceptación: 18-dic-14
Abstract
The development of heterogeneous catalytic processes is highly desirable in order to overcome some drawbacks of homogeneous catalysts, such as the separation and recycling of these catalysts. In this work, we report that Jacobsen type catalysts are efficient and selective for the oxidation of R-(+)-limonene to its corresponding diepoxides using in situ generated dimethyldioxirane (DMD) as oxidant. It was demonstrated that the solid NaHCO3 addition to the initial reaction mixture, improves the catalytic activity. Additionally, catalyst samples can be recovered and reused at least twice without significant loss of its initial catalytic activity.
Key words: R-(+)-Limonene, Jacobsen Type Catalyst, Diepoxides, Catalyst Recovery.
Resumen
El desarrollo de procesos catalíticos heterogéneos es muy deseable dado que se pueden superar las dificultades que presentan los catalizadores homogéneos, tales como la separación y el reciclado de estos catalizadores. En este trabajo, reportamos que los catalizadores tipo Jacobsen son eficientes y selectivos para la oxidación de R-(+)-limoneno a sus correspondientes diepóxidos cuando dimetildioxirano generado in situ es utilizado como agente oxidante. Se demostró que la adición de NaHCO3 sólido, al inicio de la reacción, mejora la actividad catalítica. Además, el catalizador puede ser recuperado y reutilizado al menos dos veces sin pérdida significativa de su actividad catalítica inicial.
Palabras clave: R-(+)-limoneno, Catalizadores tipo Jacobsen, Diepóxidos, Recuperación del catalizador.
1. Introduction
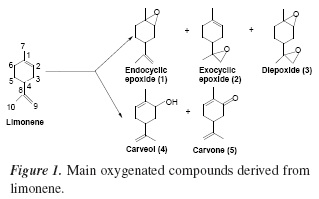
Oxyfunctionalization of limonene is of great importance for the synthesis of fine chemicals, pharmaceuticals and flavours [1]. In general, this reaction yields a variety of products (Figure 1).
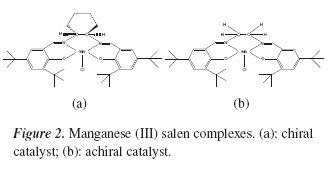
Transition metal Schiff base complexes are active homogeneous catalysts in olefin oxidation and manganese (III) complexes with salen type ligands have shown to be highly effective, chemoselective and stereoselective for this reaction [2]. Particularly, the Jacobsen type catalysts are the most popular. However, oxidative degradation of the salen ligand due to the use of most common oxidizing agents (sodium hypochlorite, iodosobenzene and 3-chloroperoxybenzoic acid) and difficult separation of the catalyst are problematic issues with these catalysts [3]. Although, immobilization of the homogeneous catalysts onto a solid support may be a strategy to overcome these difficulties, the catalytic activity is often deteriorated after heterogenizing the homogeneous catalyst [4]. Catalyst separation from products can also be achieved by using the solubility of the catalyst from the reaction mixture [5]. The chiral complex known as the Jacobsen catalyst, Figure 2a, is the most effective catalytic system for these reactions [6]. On the other hand, diastereoselective transformations might be performed with an achiral catalyst. In this case the chiral induction is governed by the substrate instead of the catalyst [7]. Figure 2b, depicts the structure of the simplest achiral manganese (III) salen complex homologous to the Jacobsen catalyst; where tert-butyl groups in aromatic rings are very important for favoring the chiral communication of the substrate with the active catalytic species, in this case, oxo manganese (V) moiety [8]. Moreover, the presence of a nitrogen organic base is crucial for stabilizing the active species and for avoiding the formation of inactive µ -oxo manganese (IV) dimeric moiety [8].
In a previous work, one of the authors described that the use of in situ generated dimethyldioxirane (DMD) as oxidizing agent in the enantioselective epoxidation of cis-ethyl cinnamate facilitated product isolation and catalyst recovery without appreciable oxidative degradation of the salen ligand [9]. This was possible by lowering catalyst solubility during the reaction [9]. In this work, the diastereoselective epoxidation of R-(+)-limonene using both chiral and achiral Mn(III) salen complexes as catalysts and in situ generated DMD in homogeneous phase is examined. On the other hand, it is well known that a slightly basic reaction medium, favors the formation of DMD [10]. Normally, it is reached by using basic buffer solution, e.g NaHCO3.
2. Experimental
Ethylenediamine, 1,2-diaminocyclohexane 99% (mixture cis and trans), L-(+) tartaric acid, 3,5-ditert-butyl-2-hydroxybenzaldehyde, manganese (II) acetate, lithium chloride, R-(+)-limonene, R-(+)limonene oxide cis/trans mixture, n-decane, Oxone® (2KHSO5•KHSO4•K2SO4), sodium bicarbonate, ethanol, acetone, dichloromethane and acetonitrile were purchased from Aldrich and used without further purification.
2.1. Catalyst Preparation
Catalysts were prepared according to the method reported by Jacobsen group [11]. The chiral catalyst (Figure 2a) was prepared in three steps. In the first step, racemic resolution of 1,2-diaminocyclohexane (mixture cis and trans) with L-(+) tartaric acid gives (1R, 2R)-(+)-1,2-diaminocyclohexane L-tartrate (chiral amine). Subsequently, salen ligand was prepared by condensation of 2.0 mmol of 3,5-di-tertbutyl-2-hydroxybenzaldehyde with 1.0 mmol chiral amine to afford (R,R)-(−)-N,N'-bis(3,5-di-tertbutylsalicylidene) cyclohexane-1,2-diamine (salen ligand). Finally, the salen ligand was treated with manganese (II) acetate in the presence of bubbling air and lithium chloride at reflux temperature (ethanol). Synthesis of achiral catalyst (Figure 2b) was carried out in the same way as chiral catalyst, but without the racemic resolution step. In this case, ethylenediamine was used as amine precursor. Both catalysts were stored without any protection against air prior to catalytic tests.
2.2. Catalyst Characterization
TGA was carried out on powder samples using a TGA 2950 with a heating rate of 2 K/min in the presence of ambient air. IR spectra were recorded using a Nicolet Avatar 330 spectrometer by the KBr technique.
2.3. Catalytic Tests
Reactions were performed as follows [12]: 30 mL acetone was added to a mixture of 5 mmol R-(+)- limonene, 0.5 mmol catalyst and 1 g sodium bicarbonate at room temperature. The pH of the resulting mixture was adjusted between 9.0 - 9.5 adding a sodium bicarbonate aqueous solution (5 wt-%). Subsequently, a solution containing the required amount of Oxoner® (2KHSO5•KHSO4•K2SO4) in 25 mL of water was slowly added during 20 to 45 minutes under continuous stirring. The pH was controlled between 8.0 and 8.5 with aqueous NaHCO3 (5 wt- %). After total addition of the oxygen source, stirring was stopped and the obtained solid filtered off. The liquid phase was treated with 30 mL of dichloromethane or acetonitrile and two liquid phases were obtained. The aqueous phase was discarded, while catalyst and reaction products were separated from the organic phase by a short-path distillation process at 0.08 MPa and 393 K. The recovered catalyst was dissolved in 30 mL acetone prior to reuse. On the other hand, the catalyst free of organic phase was dried with anhydrous sodium sulfate and concentrated under vacuum at room temperature. Then, a known amount of n-decane (internal standard) was added and the resulting solution analyzed in a 3400 Varian Star gas chromatograph equipped with a DB- 1 capillary column (50 m x 320 µm x 1.20 μm). All experiments have been repeated twice. The conversion, selectivity and diastereomeric excess was calculated by the area normalization method.
3. Results and Discussion
4. Catalyst Characterization
4.1. Thermal Analysis
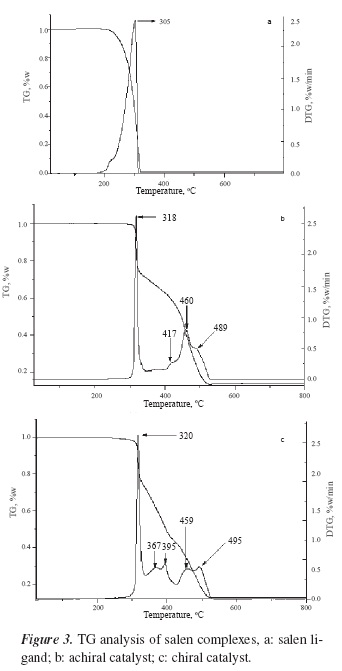
The identity and purity of synthesized salen complexes was first investigated by TGA. The salen ligand reveals a well defined decomposition temperature at 305 °C (Figure 3a). In contrast, manganese salen complexes exhibit several steps of decomposition (Figure 3b and 3c).
Thus, the achiral catalyst shifts the main decomposition temperature to approximately 318 °C, while chiral catalyst to approximately 320 °C. In both catalysts, one or two small weight loss steps are observed in the range 400-470 °C, which can be ascribed to decomposition of more stable groups (tert-butyl groups). In addition to that, a small unburned residual mass above 550 °C may be attributed to the formation of manganese oxides. Similar thermal behaviour has also been found for Jacobsen's catalyst [13].
4.2. FT-IR
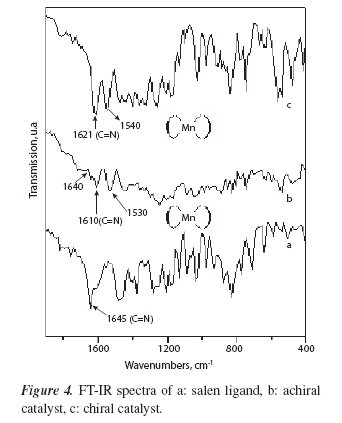
Figure 4 shows FT-IR spectra of salen complexes. The C=N imine vibration signal in the salen ligand at 1645 cm−1 (Figure 4a) is shifted in the achiral and chiral catalyst to a lower wavenumber 1610 cm−1 (Figure 4b) and 1621 cm−1 (Figure 4c), respectively. These results suggest that the imine group changes from a 2-coordinate environment in the salen ligand to 3-coordinate environment in the organomanganese complexes [13]. Moreover, the most characteristic signal from the point of view of the structure of metal salen complexes is the presence of a band between 1530-1540 cm−1 (Figure 4b and 4c), which confirm that manganese (III) salen complexes were obtained [13]. However, the achiral catalyst shows a weak signal at approximately 1640 cm−1, which may indicate that complexation with manganese ion, was not complete.
4.3. Catalytic Activity
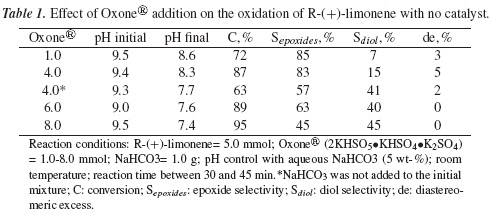
Results of catalytic experiments are presented in Tables 1 and 2. Table 1 lists R-(+)-limonene conversion vs the amount of Oxoner® added to reaction mixture without catalyst. R-(+)-limonene conversion was favored by increasing the oxygen source. However, epoxide selectivity diminished. This result can be ascribed to pH decrease with Oxone®/H2O addition. It is recognized that R-(+)-limonene oxides are very unstable under acidic conditions. Therefore, the corresponding diol is preferably formed [14]. With no catalyst, the best results were 87% conversion, 83% selectivity and 5% de (selectivity to (+)-cis-1,2 limonene oxide), which corresponds to a R-(+)-limonene/ Oxone® molar ratio of 5/4.
At the beginning, pH of reaction mixture was kept between 9.0-9.5 in order to neutralize the acid strength of Oxone® aqueous solution (pH < 1). It is known that high pH values promote Oxone® decomposition [15]. In this study, a pH value between 8.0-8.5 during reaction was found convenient for obtaining good epoxide selectivity (80-85%). On the other hand, the presence of NaHCO3 in the initial reaction mixture has been demonstrated to be very useful for the generation of DMD in aqueous solution [16]. Table 1 shows that in the absence of NaHCO3 (added as solid), R-(+)-limonene conversion and epoxide selectivity were tremendously affected.
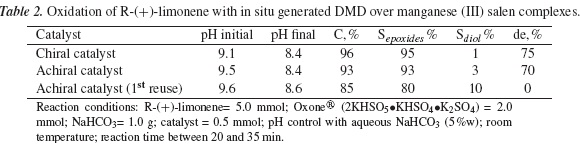
Table 2 lists conversion of R-(+)-limonene and selectivity to epoxide with in situ generated DMD over manganese (III) salen complexes of chiral and achiral manganese (III) salen complexes.
It can be noted that for obtaining a similar conversion, higher R-(+)-limonene/Oxone® (5/2) molar ratio was used as compared to that for the un-catalyzed reaction (5/4). Better selectivities to epoxide were reached indicating the positive effect of manganese (III) salen complexes as catalysts in comparison to the uncatalyzed oxidation reaction. However, the most important fact is that both catalysts, chiral and achiral, afford good diastereoselectivity to (+)-cis1,2 limonene oxide and their catalytic activities are very similar, suggesting that the induction chiral is governed mainly by substrate. This result is very important, since the amine racemic resolution step is not required for the preparation of achiral catalyst. To the best of our knowledge, activity of achiral manganese(III) salen complexes in the diastereoselective epoxidation of monoterpenes has not been reported so far. It can also be seen in table 2 that re-used achiral catalyst reaches the same conversion and selectivity than uncatalyzed reaction. However, the initial diastereoselectivity was lost. It suggests that the catalyst was deactivated, although catalyst physical loss and thermal degradation during catalyst separation process (distillation under vacuum) can not be discarded.
5. Conclusions
Achiral and chiral manganese (III) salen complexes were prepared by conventional methods. Good diastereoselectivities were reached with both catalysts, suggesting that chiral induction is governed mainly by substrate. The presence of NaHCO3 in the initial reaction mixture was found to successfully improve epoxide stability. Catalyst lost its initial diastereoselectivity, presumably by oxidative degradation.
Acknowledgments
The authors are grateful to Dirección de Investigaciones (DIN) de la Universidad Pedagógica y Tecnológica de Colombia for the financial support through contract SGI 1281.
References
[1] L. Saikia, D. Srinivas and P. Ratnasamy, "Chemo-, regio- and stereo-selective aerial oxidation of limonene to the endo-1,2-epoxide over Mn(Salen)-sulfonated SBA-15", Appl. Catal A: General, vol. 309, pp. 144-154, 2006. [ Links ]
[2] A. R. Silva, C. Freire and B de Castro, "Jacobsen catalyst anchored onto an activated carbon as an enantioselective heterogeneous catalyst for the epoxidation of alkenes", Carbon, vol. 42, pp. 3027-3030, 2004. [ Links ]
[3] Q.-H. Xia, H.-Q, Ge, C.-P. Ye, Z.-M. Liu and K.-X. Su,"Advances in Homogeneous and Heterogeneous Catalytic Asymmetric Epoxidation", Chem. Rev., vol. 105, pp. 1603-1662, 2005. [ Links ]
[4] K. Ding, Z. Wang, X. Wang, Y. Liang and X. Wang, "Self-Supported Chiral Catalysts for Heterogeneous Enantioselective Reactions". Chem. Eur. J., vol 12, pp. 5188-5197, 2006. [ Links ]
[5] C. Baleizão and H. García, "Chiral Salen Complexes:? An Overview to Recoverable and Reusable Homogeneous and Heterogeneous Catalysts", Chem. Rev., vol. 106, pp. 3987- 4043, 2006. [ Links ]
[6] J. F. Larrow and E. N. Jacobsen, "Asymmetric Processes Catalyzed by Chiral (Salen)Metal Complexes", Topics Organomet. Chem., vol. 6, pp. 123-152, 2004. [ Links ]
[7] R. A. Sheldon, Chirotechnology: Industrial Synthesis of Optically Active Compounds, Marcel Dekker Inc: New York, pp. 1-37, 1993. [ Links ]
[8] N. S. Venkataramanan and S. Rajagopal, "Effect of added donor ligands on the selective oxygenation of organic sulfides by oxo(salen)chromium(V) complexes", Tetrahedron, vol. 62, pp. 5645-5651, 2006. [ Links ]
[9] J. Cubillos and I. D. Montilla and C. Montes de correa, "Easy separation and reutilization of the Jacobsen's catalyst in olefin oxidation", Appl. Cat. A., vol. 366, pp. 348-352, 2009. [ Links ]
[10] J. Cubillos, E. Grajales, S. Vásquez and C. Montes de Correa, "Immobilization of Jacobsen type catalysts on modified silica", Rev. Fac. Ing. Univ. Antioquia, vol. 57, pp. 38-48, 2011. [ Links ]
[11] J. F. Larrow and E. N. Jacobsen, "A Practical Method for the Large-Scale Preparation of [N,N'-Bis(3,5-di-tertbutylsalicylidene)-1,2cyclohexanediaminato(2-)]manganese(III) chloride, a Highly Enantioselective Epoxidation Catalyst", J. Org. Chem., vol 59, pp. 1939-1942, 1994. [ Links ]
[12] J. A. Cubillos., PhD. thesis, RWTH University Aachen, 2005. [ Links ]
[13] L. Frunza, H. Kosslick, H. Landmesser, E. Höft and R. J. Fricke, "Host/guest interactions in nanoporous materials I. The embedding of chiral salen manganese(III) complex into mesoporous silicates", J. Org. Catal A., vol 123, pp. 179187, 1997. [ Links ]
[14] D. Steiner, L. Ivison, C. T. Goralski, R. B. Appell, J. R. Gojkovic, and B. Singaram, "A facile and efficient method for the kinetic separation of commercially available cis-and translimonene epoxide", Tetrahedron: Asymm., vol 13, pp. 2359-2363, 2002. [ Links ]
[15] P. Kachasakul, S. Assabumrungrat, P. Praserthdam and U. Pancharoen, "Extractive reaction for epoxidation of cyclohexene to cyclohexene oxide using dioxirane in ketone/Oxone® system" Chem. Eng. J., vol 92, pp. 131-139, 2003. [ Links ]
[16] H. M. C. Ferraz, R. M. Muzzi, T-deO. Vieira and H. Viertler, Tetrahedron Lett., vol. 41, pp. 5021-5023, 2000. [ Links ]