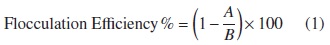Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ciencia en Desarrollo
versão impressa ISSN 0121-7488
Ciencia en Desarrollo vol.6 no.1 Tunja jan./jun. 2015
Flocculatin with Chitosan of Microalgae Native of the Colombian Plateau
Floculación con quitosano de las microalgas nativas de la altillanura Colombiana
L. M. Morenoa,*
E. Muñoz Prietob
H. Casanovac
a*Grupo GIQTA, Escuela de Química, Universidad Pedagógica y Tecnológica de Colombia, Uptc, Tunja, Colombia. Autor de correspondencia: lmmb70@gmail.com.
bGrupo DANUM, Escuela de Química, Universidad Pedagógica y Tecnológica de Colombia, Uptc, Tunja, Colombia.
cGrupo COLOIDES, Universidad de Antioquia, Medellín, Colombia.
Abstract
Microalgae are an attractive feedstock for biofuel production. Low harvesting cost upholds the use of flocculation as initial dewatering step. Two freshwater microalgae (Chlorell a sp. and Scenedesmus sp.) native from the Colombian plateau, with low/medium biomass concentrations, were selected for this study. The effects of pH, Z-potential and flocs size in dictating the behavior of chitosan as flocculant, were evaluated. This study found that the optimal flocculation efficiency of microalgae was determined at pH 7.0; besides the zeta-potential was positively correlated with the flocculant dose. The zeta-potential increases positively with a flocculant dose. The Chlorella sp. is smaller than the Scenedesmus sp. but requires a little more dose of flocculant; this aspect is due to the nature of the flocculant solution and not the size of the studied microalgae. It was observed that for Chlorella sp., chitosan coagulation shifted the flocs size from 2-4 µm to 70-80 µm, with 1.0 ml of the 40 ppm chitosan solution. The flocculation with chitosan can yield compact flocs and accelerate the settling. For Scenedesmus sp. the flocs size was shifted from 3-4 µm to 60-70 µm and less percentage in the flocs volume. Flocculation response of the microalga Scenedesmus sp. is different in comparison to that of Chlorella. The flocculant dose required is greater, although the percentage of flocculation is also higher and the flocs size is only slightly larger. Further work is needed to confirm these observations.
Key words: Flocculation, Microalgae, Chitosan, Z-Potential.
Resumen
Las microalgas son unas atractivas cepas de pienso para la producción de biocombustibles. Los bajos costos para cosecharlas, soportan el uso de la floculación como paso inicial para la extracción del agua. Dos tipos de microalgas de aguas frescas: Chlorella sp., Scenedesmus sp., nativas de la meseta colombiana, con una concentración de biomasa baja/media, fueron seleccionadas para este estudio. Se evaluaron los efectos potenciales del pH, Z y los tamaños de los flóculos, en la determinación del quitosano como floculante. Este estudio halló que la eficiencia óptima para la floculación de las microalgas se logra con un pH 7.0; además, el potencial zeta fue correlacionado positivamente con una dosis del floculante. La Chlorella sp., es menor que la Scenedesmus sp., pero requiere una dosis un poco mayor de floculante; este aspecto se debe a la naturaleza de la solución floculante y no al tamaño de las microalgas estudiadas. Se observó que para la Chlorella sp., la coagulación del quitosano cambiaba los tamaños de las madejas, de 2-4 µm a 70-80 µm, con 1.0 ml de la solución de quitosano 40 ppm. La floculación con quitosano puede producir flocs compactos para una operación más rápida. Para la Scenedesmus sp., las dimensiones de los flóculos cambiaron de 3-4 µm a 60-70 µm y menor porcentaje en el volumen de los flocs. La respuesta de floculación de la microalga Scenedesmus sp., es diferente a la Chlorella. La dosis requerida de floculante es mayor, aunque el porcentaje de floculación es más elevado y las dimensiones de los flóculos solo son un poco mayores. Se requiere más trabajo para confirmar estas observaciones.
Palabras clave: Floculación, Microalgas, Quitosano, Potencial-Z.
1. Introduction
World population growth and improved standards of living in developing economies, imply new initiatives to change the economy from a fossil-fuel-based one, to another bio-based, a part of it will be, that petroleum will be replaced by biomass [1]. A remarkable source for biofuel production, and today disregarded, is the micro algae biomass; however, its crop recovery means, i. e. harvesting, to assume for at least a 25% of the total biomass production cost, due to the highly diluted nature and the small size of microalgae culture.
Flocculation is one of the preferred techniques for harvesting microalgae, because of its simplicity and relative low cost. It is preferred to other traditionally used techniques such as centrifugation, sonication, filtration and coagulation. Flocculation methods result in higher particle sizes that enable gravity sedimentation, centrifugal recovery as well as filtration [2]. Flocculation is an effective process, that allows rapid treatment with great quantities of microalgae cultures [3]. Flocculation is the coalescence of separate suspended microalga cells into larger attached conglomerates. Firstly, the cells are aggregated into greater particles, via the interaction of flocculants with the surface charge on the cells. Then, the aggregates coalesce into large flocs that settle out of suspension [4]. A large number of chemical products have been tested as, flocculants, including various inorganic multivalent metal salts [5] and organic polymer/polyelectrolytes [6]. In addition, recently some microbes have been applied to flocculating certain microalgae [7-9].
Harvest of medium or large-scale cultivation of algae, by flocculation, is a more convenient process than contemporary methods such as centrifugation or filtration, and allows the treatment of large quantities of microalgae [10], besides can be applied to a wide range of species [11]. Different flocculants ha-ve been used for microalga harvesting. Among them, aluminum and ferric salts, which are preferred due to their high efficiency and suitability of forming flocs with microbial cells, such as those of microalgae. Aluminum Sulphate (Alum) is most widely used for removal of algae, because of ease application [12, 13]. However, it cannot be applied over a wide pH range. Moreover, flocs size with alum when compared to ferric flocs is smaller, resulting in ineffective sedimentation [12]. Although Alum (hydrated aluminum potassium sulfate) and other aluminum salts are widely used as flocculants, for sewage dewatering and for removal of algae from drinking water, are undesirable for animal feed unless the aluminum is removed [14]. Some cations such as calcium and magnesium also have a positive effect on flocculation at high pH [15]. In addition, cationic polymers such as chitosan [16] or alkalis such as NaOH have been used to achieve better flocculation. However, in spite of that, chitosan is a very efficient flocculant. It works only at low pH, but pH in microalga cultures is relatively high [17]. An alternative to chitosan is a cationic starch, which is prepared from starch by addition of quaternary ammonium groups. The charge of those quaternary ammonium groups is independent of pH and therefore, cationic starch, works over a broader pH ranges than chitosan [6]. Other examples of biopolymers than can be used to flocculate microa[gae are poly-γ glutamic acid [18] or carbohydrates as chitosan and polyacrylamide polymers [19]. A general problem of polymer flocculants is that they undergo coiling at high ionic strengths and become ineffective [20]. Therefore, they are less suitable for harvesting microalgae cultivated in seawater. Alkaline iron III hydroxides may also be used as a flocculant but has some toxicity problems. Toxic flocculants are also unacceptable because they do not allow the whole algae or residues after oil extraction to be used as feed, or as feedstock for further fermentation.
Without considering its relatively high price, an adequate alternative to overcome these limitations is to use natural polymers such as chitosan. This is a linear poly-amino-saccharide, obtained from deacetylation of chitin. Chitosan is soluble in acids but insoluble in water, has a viscosity of 20-280 centipoises, a molecular weight of 5-19 X 104, a density of 0.15-03 g.cm−3 and a deacetylation degree of 7585%. Besides, chitosan has high flocculation ability, low dose requirements for harvesting, non-toxic immediate effects on downstream applications for fish and animals, among others.
There are several studies related with the concentration of microalgae, and the most adequate amount of flocculant required for the best flocculation results. It has been assumed that there is a direct, linear, stoichiometric relationship between the number of algal cells and the amount of flocculant required no matter what the concentration of algae. As a part of such studies, for instance, the relationship between an aluminum flocculant and the zeta potentials of dilute freshwater algae, and Cyanobacteria was studied. The zeta potential does not need to be reduced to zero, even in those conditions. It only needs to be sufficiently lowered so as not to inhibit surface aggregation [21].
According to this theory, the amount of flocculant required, should be a direct function of the number of algal cells except for polymeric polyelectrolytes, such as chitosan that can flocculate by "bridging" (cross-linking) between cells, It is statistically "easier" to form aggregates at higher alga densities with, cross-linking flocculants [16]. Such bridging is not expected with small molecular weight flocculants, even divalent ones. In this study, flocculation induced by the pH increase for harvesting microalgae was evaluated. Increasing the medial pH value induced the highest flocculation efficiency of up to 90% for freshwater microalgae (Chlorella sp. and Scenedesmus sp.) with low/medium biomass concentrations.
2. Methods
2.1. Microalgae Strains and Culture Conditions
Two microalgae strains from Boyacá lagoons belonging to the modified Bold Basal medium was composed of (mg/L): KH2PO4 (175), CaCl2.2H2 O (25), MgSO4.7H2O (75), NaNO3 (250), K2HPO4 (75), NaCl (250), Na2EDTA (50), KOH (31), FeSO4.7H2O(4.98), H2SO4(conc.) (1µl),H3BO3 (11), MnCl2.4H2O (1.81), ZnSO4.7H2O (0.222), NaMoO4.5H2O (0.39), CuSO4.5H2O (0.079), Co(NO3)2.6H2O (0.0494), NaOH(0.01N).
All the microalgal strains were grown in a glass photobioreactor (volume 4L) at 26 ºC, and exposed to a continuous illumination at a light intensity of 300 µmol m−2 s−1 by cool-white fluorescent lamps. The cultures were continuously aerated by gently bubbling air containing 1 % CO2 (v/v). Chitosan was obtained by Sigma Aldrich. 100 mg of dry weight Chitosan was mixed with 10 mL of water with 1 % of Acetic Acid (HAc) solution, with continuous stirring for 30 minutes. The solution was diluted to 100 mL, using deionized water to make final chitosan concentration of 1000 mg/L [22].
3. Flocculation Efficiency
After the flocculation of microalgal cells, an aliquot of culture was withdrawn and used to measure OD550 (optical density at the wavelength of 550 nm) using a UV/Vis Spectrometer Genesys 20 TM. [16, 23, 24]. The flocculation efficiency was calculated according to the following equation (Ec 1):
A: OD550 of sample; B: OD550 of reference
Zeta potential measurements were obtained using a Malvern Zetasizer 2000HSA (Malvern, UK). OD550 was measured using a Genesys 10 spectrometer (Perkin-Elmer Instruments). Microscopic pictures were taken on an optical microscope (OLYMPUS CX41RF).
Flocculation experiments were all run with small volumes of the medium (20 mL) distributed in cylindrical glass tubes (40 mL). For freshwater microalgae with low/medium biomass concentrations (dry weight ≤ 1g/L), effective flocculation was achieved by adjusting the pH with 1 M NaOH. The pH of the suspension was controlled with a Fisher Model 230 pH meter and adjusted by adding 0.2 N H2S04 or 0.1 N NaOH prior to stirring.
After the pH had been adjusted; the glass tube was vortexes thoroughly for 30 s and allowed to stand at room temperature for 10 minutes. Then an aliquot of a medium was withdrawn and used to measure OD550.
4. Results and Discussion
The pH medium affects the harvest efficacy of microalgae [25]. Using chitosan as flocculant in the Scenedesmus sample, the highest harvesting efficiency of 99 ± 0.6% (with 40 mg/L of chitosan) was obtained (table 1). A pH 7.0 was the optimal pH; this agrees with reports from different authors [6]. The pH effect can be explained by physical property of chitosan and physicochemical interactions between chitosan and microalgae cells [26].
It is well known that a change in pH affects the flocculant structure. At neutral pH, the flocculant is present in coiled like structure. At acidic pH, it forms large flocs due to more positive charge, which work as ligands. As a result, flocculation efficiency increases [27].
At pH 7.0, the zeta-potential was positively correlated with the flocculant dose. Some other authors have reported that the zeta potentials were pH dependent and negative about pH values of practical interest. For freshwater microalgal systems in so-me cases, the trends of zeta potentials, firstly, went downwards and then upwards [26]. Table 1 shows that the zeta-potential increased from −48,4 ± 0.4 mV (in control) to −25 ± 0.4 mV at 40 mg/L of chitosan, in Scenedesmus sp and from −34.2 ± 0.3 mV (in control) to −21,0± 0.3 mVat1o mg/L of chitosan, in Chlorella sp. Generally, the zeta-potential of microalgae culture increases positively with a flocculant dose. In those experiments in which the zeta potential decreases, the declining trend of zetapotential is likely due to dissociation of carboxylic acid groups of microalgae cells' surface, which generates negative ions. Wu et al. have observed the decreasing trend of zeta-potential with an increase in the flocculant dose [28] and [21]. In general, differences observed in zeta potential vs. coagulant dose curves are explained in terms of varying pH, charge density or complexation of coagulant. When an experiment is conducted at the same pH and the coagulant dose is normalized against the charge density of the algae. Hence, the various doses required to achieve a neutral zeta potential and gradient reffect a difference in coagulant interaction mechanism of the cells, particularly with respect to complexation. Nevertheless, flocculation depends on the properties of microalgal cell surfaces; these properties differ between species and vary within a species depending on culture conditions. The cell surface to biomass ratio increases with decreasing cell size. Therefore, slighter species will require a higher flocculant dose to harvest the same amount of biomass than larger species. However, in this study, Chlorella sp. is smaller than Scenedesmus sp but required a lesser dose of flocculant. Probably, this aspect is due to the nature of the flocculant solution and not the size of the studied microalgae.
The aforementioned increase in zeta-potential indicates a decrease in surface charge of microalgae cells. Positively charged amino group of chitosan decreased the repulsion and electrostatic double layer. As a result, charge neutralization occurred to flocculate the microalgae cells. It is widely accepted that microalgae cells are negatively charged, however, a local functional group on microalga cell can be positive. Ulberg and Marochko have demonstrated that during cell microalgae growth, a negative charge is accumulated inside the cells and of contrary sign outside the cell [29]. Nevertheless, inactive cells or dead cells do not have ionic transport system, and thus, surface charge is determined by the surface equilibrium charge.
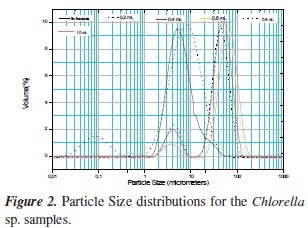
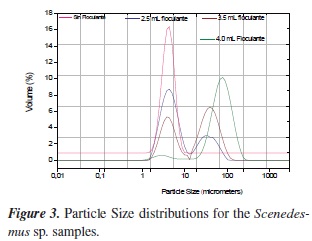
The results of the particle size distributions of the coagulated samples after 30 minutes settling with and without different concentration of flocculant for Chlorella sp and Scenedesmus sp, are shown in Figures 2 and 3. It is observed that for Chlorella sp., chitosan coagulation shifted the ï¬ocs size from 2-4 µm to 70-80 µm, with 1.0 ml of the 40 ppm chitosan solution. The flocculation with chitosan can yield compact flocs for a more rapid settling. For Scene desmus sp the flocs size was shifted from 3-4 µm to 60-70 µm and less percentage in the flocs volume.
Flocculation response of the microalga Scenedes mus sp. Is different in comparison to that of Chlorella. The flocculant dose required is greater, although the percentage of flocculation is also higher and the flocs size is only slightly larger.
5. Conclusions
The effect of chitosan as flocculant on separation efficiency of microalgae was identified; (94-99%) cell removal was achievable for both microalgae species providing sufficient coagulant addition. Found pH 7, 0 to support the highest effciency. Parameters like size distributions, Z-potential and their consequences on separation effciency, have been evaluated and studied, too. Flocculation depends on the properties of microalgal cell surfaces; these properties differ between species and vary within anyone of them, depending on culture conditions. The zeta potential at optimum removal was measured and it was observed that when the zeta potential was reduced to between â42.4 mV and â21.0 mV, removal of microalgae and some of the associated organic material was optimized, irrespective of the coagulant dose.
Acknowledgements
This work was supported by the Pedagogical and Technological University of Colombia (UPTC), Research Groups: Science and Technology of Foods (GIQTA) and Development and Applications of New Materials (DANUM) of The School of Chemical Sciences; Tunja -Colombia.
References
[1] D. Vandamme, I. Foubert, and K. Muylaert. "Flocculation as a low-cost method for harvesting microalgae for bulk biomass production", Trends in Biotechnology, 31, 4, pp. 233-239, 2013. [ Links ]
[2] E. Molina Grima, E.H. Belarbi, F. G. Acién Fernández, A. Robles Medina, and Y. Chisti. "Recovery of microalgal biomass and metabolites: process options and economics", Biotechnology Advances, 20, 7-8, pp. 491-515, 2003. [ Links ]
[3] H.-M. Oh, S. Lee, M.-H. Park, H.-S. Kim, H. C. Kim, J.-H. Yoon, G.-S. Kwon, and B.-D. Yoon. "Harvesting of Chlorella vulgaris using a bioflocculant from Paenibacillus sp. AM49", Biotechnology Letters, 23, 15, pp. 1229-1234, 2001. [ Links ]
[4] R.M. Knuckey, M.R. Brown, R. Robert, and D.M.F. Frampton. "Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds", Aquacultural Engineering, 35, 3, pp. 300-313, 2006. [ Links ]
[5] J. Duan, and J. Gregory. "Coagulation by hydrolysing metal salts", Advances in Colloid and Interface Science, 100-102, pp. 475-502, 2003. [ Links ]
[6] D. Vandamme, I. Foubert, B. Meesschaert, and K. Muylaert. "Flocculation of microalgae using cationic starch", J. Appl. Phycol., 22, 4, pp. 525-530, 2010. [ Links ]
[7] D.-G. Kim, H.-J. La, C.-Y. Ahn, Y.-H. Park, and H.-M. Oh. "Harvest of Scenedesmus sp. with bioflocculant and reuse of culture medium for subsequent high-density cultures", Bioresource Technology, 102, 3, pp. 3163-3168, 2011. [ Links ]
[8] A. Lee, D. Lewis, and P. Ashman. "Microbial flocculation, a potentially low-cost harvesting technique for marine microalgae for the production of biodiesel", J. Appl. Phycol., 21, 5, pp. 559-567, 2009. [ Links ]
[9] S. Salim, R. Bosma, M. Vermuë, and R. Wijffels. "Harvesting of microalgae by bioflocculation", J. Appl. Phycol., 23, 5, pp. 849855, 2011. [ Links ]
[10] K. Lee, Y. Kwon, and H. M. Oh. "Effects of harvesting method and growth stage on the flocculation of the green alga Botryococcus braunii", Letters in Applied Microbiology, 27, 1, pp. 14-18, 1998. [ Links ]
[11] B. Pushparaj, E. Pelosi, G. Torzillo, and R. Materassi. "Microbial biomass recovery using a synthetic cationic polymer", Bioresource Technology, 43, 1, pp. 59-62, 1993. [ Links ]
[12] J. M. Ebeling, P. L. Sibrell, S.R. Ogden, and S. T. Summerfelt. "Evaluation of chemical coagulation-flocculation aids for the removal of suspended solids and phosphorus from intensive recirculating aquaculture effluent discharge", Aquacultural Engineering, 29 1-2, pp. 23-42, 2003. [ Links ]
[13] A. Schlesinger, D. Eisenstadt, A. Bar-Gil, H. Carmely, S. Einbinder, and J. Gressel. "Inexpensive non-toxic flocculation of microalgae contradicts theories; overcoming a major hurdle to bulk algal production", Biotechnology Advances, 30, 5, pp. 1023-1030, 2012. [ Links ]
[14] N.A.: "Process for producing a naturallyderived carotene/oil composition by direct extraction from algae", US Patent, 4, 680, 314, 1987. [ Links ]
[15] D. Vandamme, I. Foubert, I. Fraeye, and K. Muylaert. "Influence of organic matter generated by Chlorella vulgaris on five different modes of flocculation", Bioresource Technology, 124, pp. 508-511, 2012. [ Links ]
[16] R. Divakaran, and V. N. Sivasankara Pillai. "Flocculation of algae using chitosan", J. Appl. Phycol., 14, 5, pp. 419-422, 2002. [ Links ]
[17] D.-J. Lee, G.-Y. Liao, Y.-R. Chang, and J.-S. Chang. "Coagulation-membrane filtration of Chlorella vulgaris", Bioresource Technology, 108, pp. 184-189, 2012. [ Links ]
[18] H. Zheng, Z. Gao, J. Yin, X. Tang, X. Ji, and H. Huang. "Harvesting of microalgae by flocculation with poly (γ-glutamicacid)", Bioresource Technology, 112 (0), pp. 212-220, 2012. [ Links ]
[19] L. Chen, C. Wang, W. Wang, and J. Wei. "Optimal conditions of different flocculation methods for harvesting Scenedesmus sp. cultivated in an open-pond system", Bioresource Technology, 133 (0), pp. 9-15, 2013. [ Links ]
[20] N. Uduman, Y. Qi, M. K. Danquah, G. M. Forde, and A. Hoadley. "Dewatering of microalgal cultures: A major bottleneck to algae-based fuels", Journal of Renewable and Sustainable Energy, 2, 1, pp. 012701, 2010. [ Links ]
[21] R. K. Henderson, S.A. Parsons, and B. Jefferson. "The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae", Water Research, 44, 12, pp. 3617-3624, 2010. [ Links ]
[22] N. Rashid, S. U. Rehman, and J.-I. Han. "Rapid harvesting of freshwater microalgae using chitosan", Process Biochemistry, 48, 7, pp. 1107-1110, 2013. [ Links ]
[23] G. Buelna, K.K. Bhattarai, J. de la Noue, and E. P. Taiganides. "Evaluation of various flocculants for the recovery of algal biomass grown on pig-waste", Biological Wastes, 31, 3, pp. 211-222, 1990. [ Links ]
[24] P. Makridis, C. Moreira, R. Alves Costa, P. Rodrigues, and M.T. Dinis. "Use of microalgae bioencapsulated in Artemia during the weaning of Senegalese sole (Solea senegalensis Kaup)", Aquaculture, 292, 3-4, pp. 153-157, 2009. [ Links ]
[25] D. J. Heasman M, W. O"Connor, T. Sushames, L. Foulkes, J. Nell. "Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs-a summary", Aquacult Res, 31, pp. 22, 2000. [ Links ]
[26] J. Morales, J. de la Noüe, and G. Picard. "Harvesting marine microalgae species by chitosan flocculation", Aquacultural Engineering, 4, 4, pp. 257-270, 1985. [ Links ]
[27] F. Renault, B. Sancey, P.M. Badot, and G. Crini. "Chitosan for coagulation/flocculation processes- An eco-friendly approach", European Polymer Journal, 45, 5, pp. 1337-1348, 2009. [ Links ]
[28] Z. Wu, Y. Zhu, W. Huang, C. Zhang, T. Li, Y. Zhang, and A. Li. "Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium", Bioresource Technology, 110, pp. 496-502, 2012. [ Links ]
[29] Z. R. Ulberg, and L. G. Marochko. "The electrophoretic properties and stability of the cell suspensions", Colloids and Surfaces A: Physicochemical and Engineering Aspects, 159, 2-3, pp. 513-518, 1999. [ Links ]