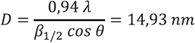1. INTRODUCTION
In the last years, the optical properties of semiconductor compounds (which in most cases are limited to TiO2, zinc oxides and cadmium) have been investigated, as well as the magnetism of ferrites, superconducting ceramics, and related compounds. In this regard, it is important to take into account that compounds with semiconductor and paramagnetic properties have not been widely studied, despite the interest in multifunctional materials, where the same compound can have two useful properties simultaneously. That is why there is a special interest on rare earth sulfides
According to different studies, sulfides belonging to the TR2S3 system (TR3+ = rare earth ion) are known to be potential materials as magnetic semiconductors, given that, they have a band gap of approximately 2.5-3 eV. An example of this is the binary compound Pr2S3, with a band gap of approximately 2.75 eV. Also, these types of materials are usually characterized by a high resistivity (p ~ 1010 Ω cm) and a wide transparency in the visible spectral range (B. B. Krichevtsov, (2000)). The presence of rare earth ions with incomplete 4f sublevels in these compounds is the responsible for the magnetic properties and the relatively large magnitude of optical effects [2].
However, the preparation of such sulfides is often a problem, because TR3+ ions are considered as strong hard acids, whereas sulfide ions are known to exhibit soft base properties, which leads to the desired compounds, being chemically unstable [1]. Known methods for the synthesis of such sulfides can be divided in two groups: first, the reaction of the rare earth oxide with carbon sulfide and second, the prior isolation of a precursor, consisting of a rare earth/ligand complex, with subsequent thermal decomposition, either in solid state or in suspension in a high boiling solvent, to give rise to the desired sulfide
For example, A. V. Selishchev et. A reports the synthesis of TR2S3 compounds, using the reaction of precursor NH2Et2 [Pr (Dttc) 4] (Ddtc = diethyldithiocarbamate) with excess carbon disulfide (CS2), giving rise to a material with semiconductor properties [1]. Although sulfur represents a less toxic alternative compared to its analogue, hydrogen sulfide, the high volatility, represents a limitation to use sulfurization as a method [3]. Additionally, another known method for the preparation of TR2S3 compounds is using the direct reaction of the rare earth oxide with CS2, however, in some cases it is possible to find oxygen incorporated as impurity in the final product, affecting the purity of the resulting phase [4].
On the other hand, Jabua, et al. (2009) reports the preparation of Pr2S3 crystalline films, by direct reaction of the elements, using thermal evaporation in a vacuum chamber, and pyroceramics and monocrystalline silicon as substrates. Although in this case the reaction is direct, and complexes are not required as precursors, the application of this method requires more sophisticated equipment, which results impractical.
Considering the importance and potential application of this type of sulfides, the current work has two purposes: first, synthesize the compound (Pr2S3) by using direct reaction of the elements, but in this case, using very simple equipment; and secondly, present the characterization of the sample, using powder X-ray diffraction. The details of such processes are described in the experimental section and analysis of results.
2. EXPERIMENTAL SECTION
Synthesis. 0.7043 g of praseodymium (rods, 99.9995%, Alfa Aesar) and 0.2405 g of sulfur (powder, 99.998%, Alfa Aesar) were mixed in a quartz tube, applying an oxygen/nitrogen flame under vacuum condition, to prevent a possible oxidation of the praseodymium along the heating. The reaction was carried out in a box type furnace and the following treatment was applied:
The tube with the mixture was heated to 200 °C (0.5 °C/min) and held for 24 h, then the reaction was raised to 400 °C (0.5 °C/min), keeping the temperature for 24 h, subsequently it was raised to 600 °C (1 °C/min), keeping the temperature constant for a period of 4 days. Once the thermal treatment finished, the sample was removed from the furnace and the tube, and then reserved for proper characterization.
Characterization by XRD. The sample obtained using the above treatment, was very well homogenized and then analyzed to determine the composition and purity, using a 2θ range between 10 and 90°. The analysis was carried out with a PANalytical X'pert powder diffractometer.
3. RESULTS AND ANALYSIS
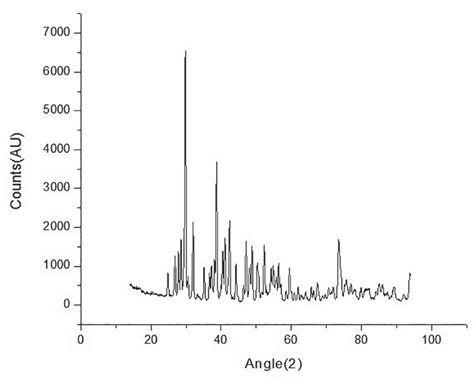
With the XRD analysis it was found that the sample actually corresponds to binary compound Pr2S3. The diffractogram that corresponds to the synthesized sulfide is shown in Figure 1.
that the diameter of the crystals in the sample corresponds to:
Based on the analysis made to the polycrystalline sample, it was found that 91.7% corresponds to the compound Pr2S3, with orthorhombic structure and space group Pnma (PDF 01-087-1643). In both cases, the diffractogram of Figure 1 and the PDF, it is possible to identify the characteristic peak of this compound (the higher intensity), corresponding to 2θ = 25, 60.
The remaining 8.3% corresponds to a small fraction of unreacted praseodymium, which is identified, according to peaks located in 29: 29.80, 34.55, 49.65 and 59.05, corresponding to the same ones in PDF 01-071-6539 (29.81, 34.56, 49.68 and 59.03). Based on these results, it could be said that binary phase obtained has a high purity. Additionally, using the Scherrer equation and the diffractogram data, it was determined that the diameter of the crystals in the sample corresponds to:
According to this result, the average particle size is much smaller compared to the crystals reported for the ligand decomposition method, where the crystal size varies between 22 and 26 nm. Considering that the band gap of the larger particles (26 nm) is 2.58 eV and for the smaller particles (22 nm) is 2.75 eV, it could be predicted that the binary compound synthesized by having a much smaller particle size (14, 93 nm) would possibly present a change in the band gap width, which would modify its properties as a semiconductor material, especially if we bear in mind that these materials have a band in the range 0.1-2 eV.
These types of compounds usually crystallize with the Th3P4 structure type, an arrangement where the praseodymium ions are located in the centers of each octahedron (formed by the ions S2-), belonging to the non-centrosymmetric point group S4. Also, Pr3+ ions occupy 8/9 of the possible central positions, while the remaining 1/9 of these sites are vacant.
Considering the complications that arise when working with sulfur, given its high vapor pressure; the simplicity of this method represents a great advantage compared to other commonly used methods, such as the reaction of rare earth oxides with sulfides, where extremely high temperatures of up to 1250 K are required [5]. The use of a direct mixture of the elements through this heat treatment (with much lower temperatures), reduces the activation energy of the compound, favoring the obtaining of the binary sulfide and consequently its application in different fields. In addition to this, by not using oxides and sulfides as CS2 or HS2 as starting reagents, (as mentioned in traditional methods) the incorporation of impurities into the resulting phase is reduced, making this method a simple and efficient option for the production of rare earth sulfides.
4. CONCLUSIONS
- The direct combination of praseodymium and sulfur, by using as thermal treatment a furnace heating and low temperatures, allows the preparation of binary sulfide Pr2S3, proving to be a practical and simple method in comparison with other traditional methods, where the use of sophisticated tools and equipment is required, in addition to very high temperatures.
-With the characterization made to the compound, it was possible to find that the achieved binary phase has a high purity, given that only a small percentage of the reagents remained un-reacted. From the grain size determined for this compound, one can conjecture that the material has a significant improvement in its semiconductor properties, in comparison with other reported materials, which have a much larger diameter.