1. INTRODUCTION
The topic of biofuels and more specifically biodiesel synthesis by transesterification reaction using oil, a catalyst and a short chain alcohol is an interesting subject. Transesterification is a process where an ester is transformed into another through interchange of the alkoxy moiety. Since the reaction is an equilibrium process, the reaction needs to be accelerated by a catalyst [1]. The use of catalysts (homogeneous acid and homogenous bases) has technical and environmental disadvantages, due to the need for neutralization and filtration processes to remove the salts formed, which generates additional costs in the separation and purification of the final products [2] [3].
Síntesis de cinamato de metilo, a través de un procedimiento de bajo impacto ambiental, y evaluación de su actividad antifúngica como potencial preservante de maderas]. In recent years, the reported works that involve the development of heterogeneous catalysts for transesterification reactions have been quite extensive, showing clearly the tendency to replace the homogeneous ones; contributing to the elimination of additional stages of the process and improving its economy [4].
The variables with the highest consideration in the transesterification reaction are: the alcohol / oil molar ratio, the amount of catalyst and the temperature, its effect being evaluated by kinetic studies and few using statistical designs. The alcohol / oil molar ratio varies from 0.1: 1 - 24: 1. The concentration of the catalyst varies from 0.25% - 6% by weight regarding to the oil. The temperatures found mostly are set at 60 °C, but the intervals are between 37 °C-75 °C. The studies in their entirety focus on controlled laboratory reactions in batch reactors with small reaction volumes (500 mL - 3 L) [5].
Most of the production studies of Biodiesel assisted by sonication are focused on the transesterification with homogeneous catalysts, being Stavarache [6] who first carried out transesterification reactions using NaOH as a catalyst, using radiation frequencies ranging between 28 and 40 kHz. The main consequences of using this technique are the reduction in reaction times, increase in the production of alkyl esters (mainly methyl and ethyl), and reduction in the amount of catalyst. Table 1 summarizes some examples of the use of Ultrasound in obtaining Biodiesel.
Table 1 Obtaining Biodiesel using catalysis and assistance with US
| Oil | Reaction conditions | Catalyst | Results | Reference |
|---|---|---|---|---|
| Palm | Frequencies of 28 - 40 kHz and the oil: alcohol ratio is 1: 6. | K2CO3 | Reaction time is reduced | [7] |
| Canola | 20 kHz, the oil: alcohol ratio was between 4-12, the temperature ranged between 45-60oC. | CaO, calcined dolomite and calcium diglyceroxide (CaDG) | Percentages in Biodiesel of 98.7%, 95.9% and 86.3% | [8] |
| Mix of Jatropha (15%), Castor (25%), rubber (20%), Cotton (25%) and frying waste (15%) | 20 kHz, the oil: alcohol ratio was 10.62, the temperature was 62.5oC. | Cu2O | [9] | |
| Frying waste | 20 kHz, the oil: alcohol ratio was 1: 4 and 1: 8, the temperature was between 40-60oC, irradiation time between 6-10 min. | KOH | Conversions of 97% | [10] |
| Frying waste | 20 kHz, the oil: alcohol ratio was 1:15, the temperature was 57oC, irradiation time was 60 min. | Hydrotalcite | Production of 76.45% of biodiesel | [11] |
| Soy | 20 kHz, the oil: alcohol ratio was 1: 3, the temperature was between 30-90oC, cata-lyst% 0.2-0.6%. | KOH | Reduction in reaction times | [12] |
| Canola and Flaxseed | 20 kHz, the oil: alcohol ratio was 1: 6 and 1: 8, the temperature was between 65oC, irradiation time was between 15-30 min. | KOH | Maximum performance of 83.4 | [13] |
| Moringa | 20 kHz, the oil: alcohol ratio was from 1: 5 to 1: 1: 7, the temperature between 40-50oC, reaction time of 60 min, catalyst concentration of 1-1.5%. | KOH | The optimum reaction temperature is close to 50 oC to 1.5% catalyst | [14] |
| Castor | 20 kHz, the oil: alcohol ratio was 1: 3 to 1: 1: 7, the temperature was 42oC, reaction time 90 min. | KOCH3 | Reduction in reaction times and good performance percentages | [15] |
2. EXPERIMENTAL SECTION
2.1 Catalysts
This study was carried out using the following solid catalysts (heterogeneous): MgCO3 (Acros Organics, extra pure), Na2CO3 (Sigma-Aldrich, ≥99.0%), K2CO3 (Sigma-Aldrich reagent grade, ≥98%, TiO2 (Aeroxide, P25) y CaO (Acros Organics, 97%). Before use, the solids are passed through a series of sieves with progressively smaller openings to guarantee a homogeneous grain size. Each catalyst was reacted with methanol and High Oleic Palm Oil -HOPO- in percentages of 1% and 3% regarding the total mass in order to carry out the transesterification of the triacylglycerides of palm oil in methanol (molar ratio 1:6) to obtain biodiesel using ultrasound equipment as a homogenizing medium.
2.2 Transesterification reaction
The HOPO (125 g) is mixed with an excess of methanol (25 g) for a molar ratio of 1:6 and the catalyst (1% and 3% by mass), then the mixture is homogenized by the ultrasound equipment (frequency 20kHz) performing tests of 6 and 12 minutes at a temperature of 42oC with a subsequent solution rest in order to achieve the separation of both biodiesel and glycerin produced at a minimum time of 48 hours and thus ensure a total separation. The formation of 11.96 g of glycerol1 is expected when all the oil reacts, which is a reference value for calculating the conversion percentage of each of the catalysts, comparing them with the amount of glycerin produced in each reaction [15].
The reaction products were analyzed by gas chromatography using a chromatograph Shimadzu model G14A with FID detector and dodecane (Sigma-Aldrich) as internal standard. Analyses were carried out with temperature program from 333 to 513 K (with a slope of 288 K min-1) and at 473 and final temperature was maintained for 5 min isothermally. The fatty acid methyl esters -FAMEs- C16: 0 (Palmitic), C18: 0 (Stearic), C18: 1 (Oleic) and C18: 2 (Linoleic) response factors were determined by calibration performed with standards and were used for quantify the percentage of biodiesel yield obtained in every reaction.
The experiments are divided into groups and carried out in duplicate: i) establish the influence on the reaction rate of type of catalyst and sonication factors; ii) Establish the influence on the reaction rate of reaction time, percentage of catalyst and molar ratio oil:alcohol factors. Table 2.
Table 2 Reaction conditions in the transesterification of HOPO
| Factor | Levels |
|---|---|
| A: Time | a1: 6 min |
| a2: 12 min | |
| >B: Percentage of catalyst | b1: 1 % |
| b2: 3 % | |
| >C: Molar ratio oil: alcohol2 | c1: 1:6 |
| c2: 1:20 |
3. RESULTS AND DISCUSSION
3.1 Without catalyst
In absence of a catalyst, it is not possible to obtain Biodiesel (% Conversion = 0), which implies that the lone stirring by sonication under the reaction conditions has no effect for the reaction to take place and that the presence of a catalyst that reduces the activation energy that the reaction requires and thus being able to obtain the desired product, in this case methyl esters. The reactors in which the US is used are generally installed to mix the two substances, oil and alcohol, where the ultrasonic cavitation emulsifies both reactants in about 5-15 seconds [16].
3.2 Without the assistance of sonication
Another factor of importance to evaluate, mainly when what is wanted is to observe the effect of the ultrasound in the transesterification reaction is to quantify the efficiency of the processes without sonication assistance. Table 3 shows the efficiency percentages of the different catalysts (3%) tested without sonication assistance.
Table 3 Influence of the catalyst on the transesterification reaction without US
| Test | Catalyst | Conversion (%) |
|---|---|---|
| 1 | TiO2 | 5 |
| 2 | CaO | 12 |
| 3 | MgCO3 | 45 |
| 4 | Na2CO3 | 47 |
| 5 | K2CO3 | 42 |
The use of a catalyst manages to decrease the activation energy necessary to pass through the energy barrier in order for a chemical reaction to take place. In the case of the transesterification reaction, this is best given in the presence of basic type catalysts, preferably over acidic catalysts [17] Among the solids used is the TiO2, which has more acid than basic characteristics [18] and this explains its catalytic behavior compared to the efficiency of the reaction, lower performance in the production of Biodiesel. As for the other solids, the percentage in conversion is greater than that of the TiO2 and varies according to the basicity of the solid in the following order of efficiency: K2CO3> MgCO3> Na2CO3> CaOf3.
Tanabe [19] found the following basicity values for the solids mmol of base / g solid: CaO: 0.007, K2CO3: 0.067 y Na2CO3: 0.057. The basicity of CaO can be improved after a heat treatment for 3 hours a 650oC to 0.073 mmol of base/g solid. The activity of solids correlates perfectly with their degree of basicity.
3.3 In presence of catalyst and with sonication assistance
Once the effects of using a catalyst and using ultrasound were determined separately, we proceeded to evaluate the effect of the two variables as a whole, discarding TiO2 due to its low conversion. Table 4 shows the conversion percentages of the different basic catalysts tested with assistance of US.
Table 4 Influence of US on the transesterification reaction catalyzed by basic solids
| Test | Catalyst | Conversion* (%) |
|---|---|---|
| 1 | CaO | 19 |
| 2 | MgCO3 | 68 |
| 3 | Na2CO3 | 71 |
| 4 | K2CO3 | 63 |
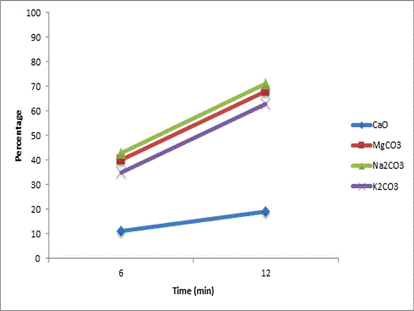
From the results, the combined effect of the catalyst in the presence of the US technique can be observed in Figure 1.
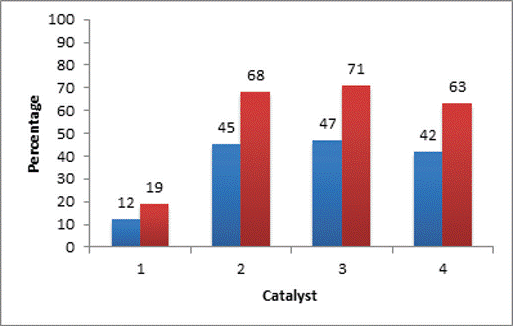
Figure 1 Effect of sonication on the transesterification reaction catalyzed by basic solids. 1. CaO. 2. MgCO3. 3. Na2CO3. 4. K2CO3. Blue squares (without sonication). Red squares (with sonication)
In the mechanism of the base-catalyzed transesterification, the first step is the reaction of the base with the alcohol producing the protonated catalyst. After that, the nucleophilic attack of the alkoxide at the carbonyl group of the triglyceride, from which the alkyl ester (biodiesel) and the corresponding anion of the diglyceride are formed. Finally, the deprotonation ofthe solid, regenerate the catalyst starting another catalytic cycle [20].
It can be seen that in all cases, the percentages of obtaining methyl ester increase, on average by 50%, with the use of sonication. As mentioned in the conceptual framework, the sonication has the capacity to increase transport phenomena of mass, since the microbubbles that form and collapse generate changes in temperature and pressure that chemically affect the molecules in the medium [4].
3.4 Reaction time effect
The time in obtaining Biodiesel through the transesterification of oils and the quality of the final product are closely related. From the previous results [15], it was decided to study the reaction at a shorter time, 6 min instead of 12 min. Table 5 shows the percentages of conversion of the different basic catalysts tested with assistance of sonication.
Table 5 Influence of US on the transesterification reaction catalyzed by basic solids. Reaction time effect
| Test | Catalyst | Conversion* (%) |
|---|---|---|
| 1 | CaO | 11 |
| 2 | MgCO3 | 40 |
| 3 | Na2CO3 | 43 |
| 4 | K2CO3 | 35 |
Percentages very close to those obtained using a catalyst in the absence of ultrasound are achieved, which indicates that the use of sonication succeeds in reducing the time the reaction takes by 50%. Figure 2 shows that over time the reaction occurs continuously, but it is noticeable that the speed is increased to a greater degree, higher slope, with the use of magnesium, sodium and potassium carbonates than that with the calcium oxide. As sonication cavitation emulsifies both reagents, oil and alcohol [16], and carbonates interact better with polar solvents than oxides [21] the combination of these two phenomena allows different reagents to diffuse more rapidly to the surface of the carbonates that made the oxide.
3.5 Effect of catalyst percentage
The catalyst percentage was evaluated, instead of 3%, it was used 1%. Table 6 shows the conversion percentages of the different basic catalysts tested with assistance of sonication.
Table 6 Influence of US on the transesterification reaction catalyzed by basic solids. Effect of catalyst percentage
| Test | Catalyst | Efficiency of the reaction * (%) |
|---|---|---|
| 1 | CaO | 10 |
| 2 | MgCO3 | 43 |
| 3 | Na2CO3 | 45 |
| 4 | K2CO3 | 40 |
* Reaction conditions: % of the Catalyst 1%, Time 12 min.
What can be seen, is that although the ultrasound manages to have a positive effect on the reaction by decreasing the times and increasing the efficiency, the presence of a catalyst is vital for the reaction to occur, as the percentage of the catalyst decreases, the percentages of yield are almost alike or less, to the reaction performed in the absence of US and only with catalyst. Figure 3 shows the comparison in the product acquisition percentages at the two different percentages of catalyst.
3.6 Effect of the molar ratio oil: alcohol
The test was done with the solid that showed the best catalytic results, Na2CO3, with a 1: 6 molar ratio of the reagents, where a percentage of biodiesel of 71% was obtained with sonication during 12 minutes, and a total conversion was achieved, 100 %, when the ratio is 1:20.
The molar ratio oil: alcohol is one of the variables that affect the conversion of triglycerides present in the oil in alkyl esters. Stoichiometrically, the transesterification process requires 3 moles of alcohol per 1 mole of triglycerides, so the excess of alcohol in the reaction is required to obtain greater speed in the process and reach a greater conversion to alkyl esters because the reaction is reversible, and the excess allows the equilibrium to shift towards the side of the products [22].
4. CONCLUSIONS
When comparing the results obtained with homogeneous catalysts NaOH type and heterogeneous as K2CO3, from the literature, it is observed that similar results are obtained which shows that this paper contributes to the use of technologies that allow to reduce the negative impacts on the environment, in the components of energy expenditure, decrease in reaction times, and the additional processes involved in the recovery of products using homogeneous catalysts.
Sodium carbonate showed the highest percentage of biodiesel production (71%), using 12h in an oil: alcohol ratio of 1: 6; so, it is intended to continue the study using this catalyst with the variation of other parameters that can similarly affect the percentages of performance such as temperature and frequency of ultrasound.