1. Introducción
The increased energy consumption demands alternative energy sources and raw materials for sustainable chemical and fuel production are being explored to reduce dependency on fossil fuels [1, 2, 3]. Levulinic acid (LA) is considered a versatile biomass-derived platform due to its two functional groups, allowing it to react as a carboxylic acid or ketone with a wide range of compounds, yielding various biofuels, polymers, and fine chemicals [1,2, 3, 4, 5,6,7, 8,9,10]. LA esters, including ethyl levulinate, find extensive use in the flavoring, solvent, and polymer industries, with the added advantage of serving as a fuel additive [13]. Ethyl levulinate offers higher engine efficiency, longer operating life, and reduced CO and NOx emissions, thanks to its high oxygen content [14, 15].
While LA esters are typically produced via esterification reactions using homogeneous catalysts like HCl, H2SO4, and H3PO4, which yield high-interest products in a short reaction time, these acid catalysts are non-recyclable, necessitating large volumes of base for neutralization, and presenting economic and environmental challenges due to equipment corrosion. The use of heterogeneous catalysts, where catalysts are easily separable and reusable, presents a more economically and environmentally viable methodology [16, 17, 18, 19, 20, 21, 22].
Various solid acid catalysts have been investigated for their potential in transforming levulinic acid to ethyl levulinate, including zeolites, resin catalysts, supported heteropoly-acids, sulfonated oxides ZrO2, or mesoporous silica or carbons with sulphonic groups. These studies indicate that LA can be efficiently transformed to levulinate esters over solid acid catalysts, provided the amount and strength of acid sites, dispersion state of active species, and surface area are appropriately optimized [23, 24, 25, 26, 27, 28, 29, 30, 31].
In recent times, there has been a shift towards the use of reusable biomass-derived catalysts, addressing issues associated with organic or inorganic catalysts prepared at the laboratory level, including high precursor costs, long synthesis times, and environmentally unfriendly methodologies. Biopolymers, such as alginate, gelatin, starch, chitosan derivatives, and cellulose, have been investigated as potential catalytic supports, offering advantages like a simple work-up process, cost-effectiveness, environmental friendliness, high product yield with purity, and shorter reaction times [32, 33, 34, 35, 36].
Cellulose, being one of the most abundant natural biopolymers globally, has garnered significant attention for its bio-degradability and renewability. Its unique properties make it an appealing alternative to conventional organic or inorganic supports in catalytic applications. Cellulose sulfuric acid (CSA) has emerged as a promising biopolymeric recyclable for acid-catalyzed reactions, including the synthesis of various compounds. CSA offers advantages such as a straightforward work-up process, cost-effectiveness, environmental friendliness, high product yield with purity, shorter reaction times, and solvent-free conditions. Furthermore, CSA is a solid, heterogeneous catalyst that can be recovered and reused multiple times without significant efficiency loss.
However, existing studies predominantly use commercial cellulose as the raw material for CSA preparation. In this study, Cortaderia Selloana, commonly known as Pampa Grass, was utilized as the starting material for cellulose extraction. Pampa Grass, an invasive species, has strong dominance potential and can adversely impact native ecosystems. Effective methods for Pampa Grass control, such as cutting or pruning, generate significant waste with no current use or value, making it a potential resource for cellulose synthesis.
This work aims to investigate the chemical conversion of levulinic acid to ethyl levulinate through esterification using CSA as a green solid acid catalyst derived from Cortaderia Selloana. To the best of our knowledge, the use of CSA as a catalyst for ethyl levulinate synthesis from LA has not been previously reported.
2. Experimental section
2.1. Extraction of cellulose
Cellulose was extracted from Cortaderia Selloana, also known as Pampa Grass, Briefly, 5 g of sample of Cortaderia Selloana plant as dried at 100 °C, and posteriorly was added a solution of H2O2, containing 200 mg of catalyst and this mixture was refluxed for 24 hours at 100 °C under mechanical stirring. The solid was dried at 40 °C obtaining a solid principally composed of cellulose.
2.2. Preparation of cellulose sulfuric acid
The cellulose sulfuric acid was obtained by mixing the solid obtained (5 g) in 20 ml of n-hexane with 1.00 g of chlorosulfonic acid (9 mmol) which was added dropwise at 0 °C for 2 h under a nitrogen atmosphere. After the completion of the addition the chlorosulfonic acid, HCl gas was removed from the reaction vessel and then stirred for a further 2.5 h. Then, the mixture was filtered and washed with 30 ml of acetonitrile and dried at room temperature. The solid was labelled as CSA
2.3. Catalytic studies
The synthesized catalysts were studied in the esterification of levulinic acid (LA) with ethanol. This process was conducted in a round-bottom flask equipped with a reflux condenser. In a typical experiment, levulinic acid and ethanol were combined in the flask, followed by the addition of the catalyst. The effect of reaction temperature (50, 60, 70, and 80 °C), catalyst loading (50, 100, and 150 mg), and molar ratio of ethanol to levulinic acid (EtOH:LA) (10:1, 5:1, and 3:1) was investigated to optimize the reaction conditions using cellulose-SO3H as the catalyst.
The quantification of products (after separating the catalyst by centrifugation) was carried out using a Varian 3400 gas chromatograph equipped with a flame ionization detector (FID). The reaction products were analyzed with a DB1 column, helium as the carrier gas, and the following conditions: oven temperature ramped from 353 K to 573 K at a rate of 10 K min-1, and a hold at 573 K for 6 minutes. The detector, FID, operated at 493 K. Conversions and selectivities were determined by comparing the relative peak areas of substrates and products using a normalization method.
3. Results and discussion
The esterification of levulinic acid into ethyl levulinate was examined both without a catalyst, employing solely the cellulose support, and with a catalyst (CSA) for 24 hours at 353 K. The results demonstrated the following trend in the yield of ethyl levulinate across the prepared catalyst samples: CSA (92%) > Cellulose (31 %) > Blank (21 %). The progression of the reaction at different time intervals is depicted in Figure 1. It is evident that the blank experiment exhibited a relatively high reaction rate, which stabilized after 16 hours of reaction, yielding a maximum of 21 %. Additionally, when employing only cellulose, there was a marginal increase in the yield of ethyl levulinate, reaching 10% at 24 hours. It is well-established that levulinic acid esterification with alcohols can occur even in the absence of a catalyst, as the acid itself is capable of catalyzing the reaction [47]. Consequently, the blank experiment exhibited catalytic activity. However, the increase in conversion observed when using cellulose can be attributed to the presence of acid sites on the surface of the cellulose, which catalyze the esterification reaction between LA and EtOH.
When the esterification of levulinic acid was conducted with CSA as the catalyst, the catalytic activity was significantly higher compared to that presented by cellulose under the same reaction conditions, achieving a conversion of 92 % for LA. The modification of the cellulose surface with chloro-sulfonic acid, through the incorporation of sulfonic groups, enhanced the conversion of LA without compromising the selectivity towards EL. In all the tests, selectivity towards EL remained at 100% with no side products. As shown in Figure 1, CSA exhibited a higher yield of EL in comparison to cellulose. This behavior can be attributed to a synergistic effect between the acid sites of cellulose, levulinic acid, and the Brønsted sites provided by the incorporation of the sulfonic groups. Consequently, further optimization of the reaction conditions for the esterification of levulinic acid with ethanol using CSA as the catalyst was performed to maximize the yield of EL.
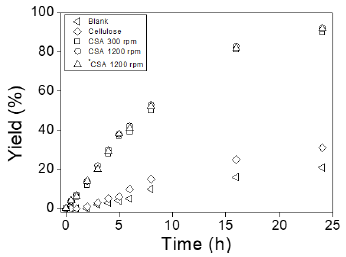
Figura 1: Esterification of levulinic acid with ethanol to ethyl levulinate. Reaction conditions: 5 mmol of LA, 50 mmol EtOH, 0.1 mg catalyst, 80 °C, and 24 h.
The effect of the molar ratio of LA to ethanol on LA esterification to EL with respect to time is shown in Figure 2. It is known that LA esterification is a reversible reaction, and according to Le Chatelier's principle, an excess of ethanol shifts the equilibrium of the reaction towards EL formation, resulting in higher LA conversions [52]. Therefore, an excess of ethanol was employed in the reactions (molar ratio EtOH:LA ranging from 3:1 to 10:1) to favor the forward reaction of LA to EL. When the molar ratio increased from 3:1 to 5:1 and 10:1, the results showed a slight decrease in LA conversion to EL. This behavior can be attributed to the use of a large amount of ethanol, which likely affected the reaction activity by reducing the concentration of LA [52, 56]. Accordingly, the low concentration of LA also led to mass transfer limitations and low accessibility of LA for adsorption on the catalyst, thereby inhibiting the production of the corresponding ester. However, in this study, excess ethanol did not promote other side reactions such as the dehydration of ethanol and transfer hydrogenation of LA for the formation of GVL, as reported by Siva et al. [49]. When the test was repeated, very similar results were obtained, indicating the reproducibility of the experiment.
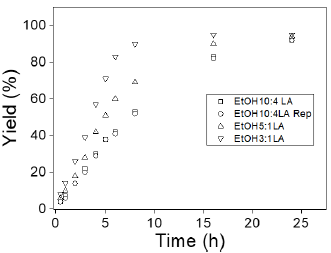
Figura 2: Effect of the molar ratio of EtOH:LA on the esterification of levulinic acid with ethanol to ethyl levulinate. Reaction conditions: 5 mmol of LA, 100 mg catalyst, and 24 h.
The impact of CSA loading, ranging from 50 to 150 mg, on LA esterification to EL is depicted in Figure 3. The LA conversion increased from 26 % to 58 % with an increase in catalyst loading from 50 to 100 mg. This catalytic behavior is attributed to the higher concentration of active sites. However, an increase from 100 to 150 mg of catalyst did not result in significant changes in the conversion of LA to EL. In all tests (50, 100, and 150 mg), the selectivity towards EL remained unchanged at 100 %. Thus, 100 mg was determined to be the optimal catalyst amount to achieve higher conversion of LA without affecting selectivity towards EL.
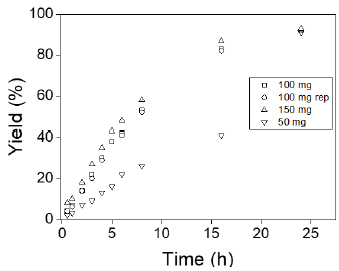
Figura 3: Effect of catalyst amount on the esterification of levulinic acid with ethanol to ethyl levulinate. Reaction conditions: 5 mmol of LA, 50 mmol EtOH, 80 °C, and 24 h.
The effect of reaction temperature was investigated at 50, 60, 70, and 80 °C, utilizing 100 mg of CSA, a reaction time of 24 hours, and a molar ratio of 10:1. It is well-recognized that the esterification reactions of fatty acids with alcohols are endothermic processes [48]. Consequently, the conversion of LA to EL is favored at higher temperatures. As illustrated in Figure 4, the conversion of LA increases with reaction temperature. The highest conversion (92 %) was achieved at 80 °C. When the test was repeated, similar results were obtained at 80 °C, indicating the reproducibility of the experiment.
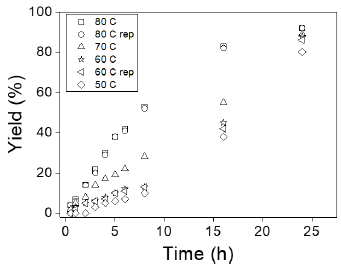
Figura 4: Effect of reaction temperature on the esterification of levulinic acid with ethanol to ethyl levulinate. Reaction conditions: 5 mmol of LA, 50 mmol EtOH, 100 mg catalyst, and 24 h.
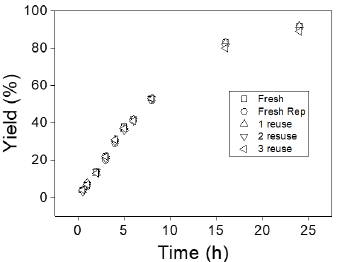
The reusability of CAS was studied over four cycles, including the use of a fresh catalyst (Figure 5). After each reaction, the CAS catalyst was recovered by centrifugation, washed with ethanol, dried overnight, and returned for subsequent reactions. The catalytic activity of CAS was observed to be stable for four runs, with a very slight decrease in EL yield from the first to the fourth run. The decrease in EL yield from the second to the fourth run is likely due to the loss of catalyst during the filtration and washing steps. Additionally, the deposition of reactant and product on the catalyst's active sites possibly resulted in a small decrease in catalyst activity [13]. The used CAS catalyst was not further characterized in this study.
4. Conclusions
Cellulose sulfonated catalyst was efficiently and environmentally prepared from residual biomass of Cortaderia Selloana and this solid was studied in the ethyl levulinate through the etherification of levulinic acid at relatively low temperatures. Our results were compared with reports utilizing inorganically supported solid acids and acidic resins. Notably, we achieved an outstanding yield of 90 % for EL, demonstrating the catalyst's remarkable performance. Additionally, the catalyst exhibited recyclability without any discernible loss in activity. The experimental procedure was straightforward, adding to the practicality of this method. Furthermore, the catalyst is non-toxic, non-corrosive, and cost-effective, enhancing its environmental and probably economic viability.