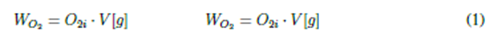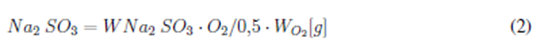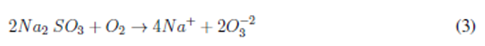Introduccion
The lack of dissolved oxygen (DO) in bodies of water (WBs) has badly affected various ecosystems [1]. This lack depends on the following aspects: the quality of water and physical, biological, and climatic factors, which may hinder the content of DO in water, thus affecting the life present in WBs [2]- [4]. To overcome this problem, several methods have been introduced for the aeration and restoration of DO in WBs, enabling the mineralization and elimination of most contaminants from water [5]- [7]. A prominent example of this is the management of aeration processes by mechanical and diffusion methods, which have allowed the transfer and regulation of DO in anoxic water.
Mechanical aeration systems have a low energy consumption compared to conventional diffusion systems [2]. However, the application of diffusion systems in aeration processes is widely accepted due to their efficiency in large BW [8]. On the other hand, the aeration processes and the treatment of BW are related to other factors such as microorganisms, organic matter, and sludge due to landslides, which transport the clays through the rainwater and that can affect the transfer processes of DO in WBs [9]. The DO in water bodies can vary due to different reasons, which may be natural or anthropogenic. The latter is related to polluting processes and the alteration of ecosystems with raw wastewater discharges. Wastewater, when discharged into water bodies, can decrease the concentration of dissolved oxygen (DO) [10], [11]. Under natural conditions, the effect of rain can cause landslides, which transport sediments and clays that can affect DO transfer processes [9]. This, in turn, is influenced by other factors such as: temperature [12], high levels of organic matter [13], depth, river turbidity, and runoff caused by rainfall [14]- [16]. These agents affect the transfer of oxygen in the water, thus increasing energy consumption during water treatment processes. This is why the elimination of some of these agents prior to performing an aeration process is preferred [8].
In several cases, the presence of clays in WBs favors the retention and elimination of elements. This is the case of adsorption heavy metals, present in WBs, due to their positive charge, thus allowing a lower energy consumption in downstream purification processes [17]. Moreover, the presence of clays can also contribute to the removal of microorganisms while increasing the oxygen content in WBs [18]. However, the aforementioned studies have not shown the influence that clays can have during DO transfer processes in clean BWs with an oxygen deficit. The objective of this work is to study the influence of clays present in the Paipa-Boyacá region in Colombia on the transfer of DO in samples of oxygen-deficient waters (anoxic waters) produced on a laboratory scale.
Materials and experimental details
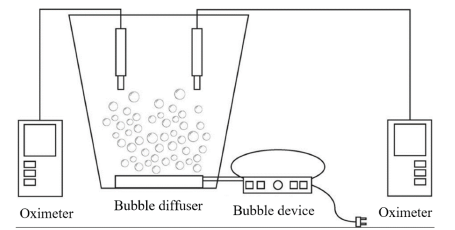
Measurements were made on 12 L samples of clean water with 5 repetitions per sample, for a total of 20 trials. In prior tests, the samples were treated by distillation to ensure the elimination of biological and particulate material. The initial dissolved oxygen was measured by using a Hachflexi HQ30d US Pat (6912050) oximeter at a height of 2800 m.a.s.l and a pressure of 740 hPa. The oximeters were placed inside the assembly, as shown in Fig. 1. The DO data obtained in water samples as an initial parameter was 7,32 g·L-1. This parameter allowed establishing the total dissolved oxygen in the water samples using Eq. (1). All tests were carried out at a constant temperature of 17 °C, giving greater importance of the study to the influence of the presence of clays on the transfer of DO in water.
The elimination of DO in the water was done by adding sodium sulfite (Na2SO3), as presented in Eq. (2), and a catalyst cobalt chloride was used [6]:
where Na2 SO3 is the molecular weight of sodium sulfite required in [g] to remove all the dissolved oxygen present in water samples, O is the molecular weight of oxygen [g], WNa2 SO3 is the molecular weight of sulfite in one mole of Na2 SO3, WO2 is the total dissolved oxygen content in each of the water samples in g, O2i is the DO content [g·L-1], and V is the water volume [L]. This study used an additional 0,5% sodium sulfite during the process to ensure the total elimination of the DO present in each of the water samples.
The use of the sulfite technique for the elimination of DO in this study allows a stable control system for each of the treated samples. The application of sulfite favors the elimination of the DO present in water by through te formation of sulfate ions, which is described by Eq. (3). To accelerate the kinetics of the reaction, CoCl 2 was used, thus ensuring that this reagent does not influence DO transfer [6]
The anoxic water samples were prepared with bentonite clays obtained from the study area. The study was carried out by adding 0, 1, 3 and 5% of the additional weight of bentonite in the anoxic water samples, making 3 replicates for each type of sample. The samples were stirred for 20 minutes to ensure the homogeneity of the clays in the solution, as well as the removal of clusters from the clay during deposition into the water samples. 5 replicates were made for each type of sample, for a total of 20 samples in this study.
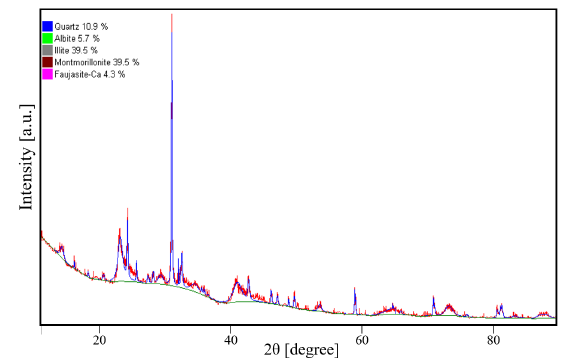
The clay samples were obtained in the municipality of Paipa, Boyacá, Colombia. The material was sieved at 74 microns (200 meshes) and dried in an electric oven at 105 °C for 6 hours. To characterize the clay samples, they were homogenized and analyzed using the X-ray diffraction (XRD) technique with a Phillips PW1700 X-ray diffractometer, equipped with a 26° graphite monochromator, a Co source at wavelength λ= 1,75 Å, a PW1825 generator, and a Pixel variable angle detector with the Bragg-Brentano configuration [19]. The diffraction pattern is showed in Fig. 2, as well as the result of the clay composition analysis obtained through XRD. The characterization of the sample was performed using the HighScore-Plus software, supplied by PANanalytical X’pert Pro. The samples have a main composition of montmorillonite and illite (~79% of the total composition), which may be closely related and can contain other compounds in a lesser proportion, such as quartz and other silicate-type minerals [20], [21]. This study found that the clay samples used are composed of quartz, albite, and faujasite occurrents from the zone [22]. The XRD pattern shows a characteristic diffraction peak for quartz at an angle of 31°. The diffraction peaks generated for montmorillonite and illite are consistent with those reported by [23] using a source of CuK∝1 L3 (λ= 1,540598 Å). The transfer of the diffraction peaks is due to the type of source used.
The DO transfer process was carried out through a diffuse vertical bed aeration system, using a 16 W air pump with a 2,5 L·min-1 air transfer and a bubble membrane. The management of this type of aeration systems allows a better control of the variables that occur during theWBrestoration and DO transfer processes. Restricting operations to a system with vertical aeration, such as the one presented in Fig. 1, gives greater control over the DO transfer process, which allows to eliminate variations in the data due to the horizontal currents or flows within each system [24]. The tests were performed until a constant DO content was obtained. The data obtained in the DO transfer process for each of the measurements allowed determining the energy consumption and the efficiency of the aeration process using the calculations reported by [8]
Results
The results obtained in this research are presented in the form of graphs for an optimal relation of the data obtained from each one of the analyzed samples. These graphs allowed us to observe the characteristics and behavior of the samples during the DO transfer process in each of the tests performed. The data obtained in this study were averaged, presenting a hatch of standard variation of ~0,5% on the contribution of DO in the water samples for all the studied cases.
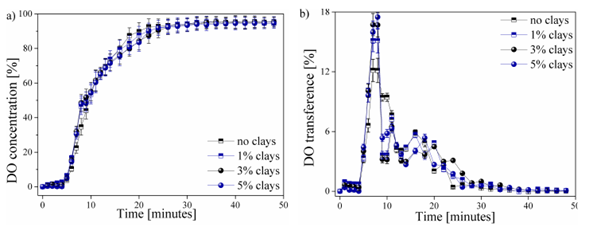
The behavior of the DO transferred in each of the water samples worked as a function of time, as shown in Fig. 3. This process can be presented in the form of the percentage of DO shrinkage ( %) until it reaches the saturation point (Fig. 3a), or in the form the percentage of DO transfer ( %) supplied in each of the time intervals (Fig. 3b).
Analysis
The behavior of the DO in the 4 studied types of water samples during the aeration process is shown in Fig. 3. Fig. 3a shows the DO concentration as a function of time up to a stable DO value. The contribution of DO transfer within time intervals is shown in Fig. 3b. Other factors that have an influence during DO transfer in water, such as temperature, were not considered.
A stagnation zone of DO content occurs during the first 5 minutes of the aeration process. This behavior is reported by all the analyzed samples. The generated transfer stagnation is due to the application of an excess of sodium sulfite, which has been mentioned previously, thus guaranteeing the total removal of the oxygen present in the initial sample [25]. No variation is observed in the samples within this range, which rules out the influence of clays.
Similarly, a behavior in the DO transfer is presented in Fig. 3a for the 4 samples. Slight variations in samples were observed between the 5-10 and the 18-24-minute intervals. These areas are best visualized in Fig. 3b, where abnormal behavior can be observed. A greater contribution to the DO transfer rate was observed due to the increase in the content of clays in the water samples, thus obtaining a maximum peak of 17,5% of the total DO transferred for the sample with an addition of 5% of the clays present in the region of Paipa, Boyacá. This transfer peak decreased as the content of clays in the water was reduced, obtaining values of 17, 15,2, and 12,1% of DO, respectively, for the samples with 3, 1, and 0% (free of clays). The possible affinity between oxygen and particles can improve the transfer rate of DO in water [26].
The behavior of the process of transfer and saturation of DO in water samples in this study could be compared with the studies realized by [5]. This behavior is of the Arrhenius type, in which the oxygen content in the sample will gradually approach without reaching the maximum theoretical value. This behavior is also seen in the DO content with respect to the variation of the temperature, evincing an inverse behavior to that of the DO transfer. [27] reported the reduction of the DO transfer rate from the 10th minute of measurement and a saturation point from the 28th, obtaining a maximum saturation value of 95 ± 1,2% for each of the 4 studied samples. The decrease in the contribution rate of DO in the water samples from values close to the saturation point has also been reported by [28] and [29], who reported that the presence of high DO values, i.e., with a distance less than the maximum point of saturation or stagnation, which requires a greater amount of input time and of oxygenation in the water to reach this saturation point.
The data presented in Fig. 3b shows that the addition of and increase in the percentage of clays in the water samples does not exert a significant influence on the maximum saturation point and the DO transfer time. However, a slight decrease in DO concentration was observed between 16-20 minutes of measurement due to the addition and increase of clays in the water samples. This behavior was observed after reaching a DO concentration. The measurements were made in controlled environments. Therefore, they are not fully reproducible on a real scale in natural water bodies. There is a hatch of variables, such as temperature, dissolved metals, microorganisms, and so on, which also affect the DO transfer process in the water. However, the obtained results are presented as an initial stage prior to the study of the effect of these clays in environments outside laboratory conditions, as well as the study of other factors that affect water quality and DO content in water. These variables are expected to be analyzed in future research.n of 80 ± 5% (Fig. 3a). Increasing the suspended clay particles can reduce the total transfer area, thus generating a decrease in the contribution of DO at this stage of the process, i.e., generating a surface contamination that allows the contribution of DO to the WB [30].
The value of the coefficient at room temperature of the oxygen transfer (K La·h-1) shows an average value of 12,45 ± 3,28 a.u.With this value, it is possible to obtain an average value of N of 0,86 ± 0,12 kg O2·kW-1·h-1 for each of the samples in this study. This value remains within the range of efficiency in the aeration processes by diffusion mechanisms (from 0,3 to 1,2 kg O2·kW-1) [8], showing that the system behaves in conditions similar to those found at the industrial level. The values of the standard oxygen transfer rate (SOTR) and standard oxygen transfer efficiency (SOTE) are 12,31 kg O2·h-1 with an efficiency of 38,48 %. Therefore, the energy consumption of each of the samples remained within the range established by the literature [8], [31], [32]. These values can vary with pressure generated by the flow of the gas that transports oxygen, as well as the height of the water column (depth) [33]. This opens a study hatch to analyze the effect of these parameters in future work.
From a general point of view, the study shows the following:
the presence of clays in anoxic water bodies favors the required DO transfer;
the presence of clays in water bodies with a lower DO content, but close to its saturation point in the water, shows a harmful behavior in reducing the DO transfer capacity in water; and
the variation and increase of clays in cases 1) and 2) affects the processes of DO transfer.
This is favorable for water bodies lacking DO (anoxic), while a low or null content of clays in bodies of water with a relatively high DO content but it produces a lower than the saturation point of the DO in water. Our results are consistent with previously reported work [34], which presented the low influence of clays during the transfer of DO in bodies of water. The presence of clays exerts a degree of influence on the DO transfer process in a superficial way.
These measurements were made on a laboratory scale, so they should be taken as an initial phase that must be tested with other variables that affect DO concentration, considering the characteristics of the natural water bodies to be studied. Factors such as temperature were not considered in this study; we focused only on the effect of the clay studied during the transfer of DO in anoxic water samples under controlled conditions.
Conclusions
In this study, we have shown the possible influence of the clays on WBs during DO transfer processes in a controlled environment. The data obtained in this study allowed us to establish that the presence of clays in WBs with a lack of DO (anoxic waters) does not have a direct influence on the DO saturation point during the oxygen transfer process through the use of diffuse aeration, i.e., the presence and increase of the concentration of clays present in Paipa, Boyacá, Colombia. However, the data allowed us to establish that the presence of clays in the water can favor the transfer of oxygen in the first periods of time. Therefore, this is an energy advantage for WBs with a high lack of DO and a relatively high percentage of clays. The behavior decreases as the DO content in the system increases, with a low clay content within this type of WB, which promises to be a better option.
The measurements were made in controlled environments. Therefore, they are not fully reproducible on a real scale in natural water bodies. There is a hatch of variables, such as temperature, dissolved metals, microorganisms, and so on, which also affect the DO transfer process in the water. However, the obtained results are presented as an initial stage prior to the study of the effect of these clays in environments outside laboratory conditions, as well as the study of other factors that affect water quality and DO content in water. These variables are expected to be analyzed in future research.