Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Reumatología
Print version ISSN 0121-8123
Rev.Colomb.Reumatol. vol.14 no.2 Bogotá Apr./June 2007
Artículo de revisión
Prediction and prevention of rheumatoid arthritis
Predicción y prevención de la artritis reumatoide
T. W. J. Huizinga, A. H. M. van der Helmvan Mil1
1 Department of Rheumatology, Leiden University Medical Center, The Netherlands.
Recibido para publicación: mayo 15/2007 Aceptado en forma revisada: junio 25/2007
Summary
A substantial proportion of patients who present with probable rheumatoid arthritis (probable RA or undifferentiated arthritis = UA) progresses to RA. In a randomized trial we demonstrated that in patients with UA methotrexate is an effective drug to inhibit symptoms, structural damage, and progression towards RA. However 4050% of UApatients remit spontaneously. Therefore adequate treatment decisionmaking in earlyUA necessitates identification of the UApatients that will develop RA. We developed a prediction rule using data from the Leiden Early Arthritis Clinic, an inception cohort of patients with recentonset arthritis (n=1700). The patients that presented with UA were selected (n=570); progression to RA or other diagnosis was monitored after oneyear followup. The prediction rule consisted of nine clinical variables: gender, age, localization of symptoms, morning stiffness, tender and swollen joint count, Creactive protein, rheumatoid factor and antiCCP antibodies. Each prediction score varies between 0 and 14 and corresponds to a chance (percentage) RA development. Thus, in earlyUA the risk to develop RA can be predicted, thereby allowing individualized treatment decisions to initiate diseasemodifying antirheumatic drugs in patients who present with UA.
Key words: rheumatoid arthritis, undiffertiated artritis, prediction, prevention.
Resumen
Una buena proporción de pacientes que se presentan con artritis reumatoide probable (AR probable) o artritis indiferenciada (AI), progresan a AR. En un estudio aleatorizado, nosotros demostramos que en pacientes con AI, el metotrexate es un medicamento efectivo para mejorar los síntomas, evitar el daño estructural y la progresión hacia AR. Sin embargo, 4050% de los pacientes con AI remiten espontáneamente. De esta manera, para hacer una buena decisión terapéutica en pacientes con artritis temprana indiferenciada, necesitamos identificar mejor aquellos pacientes con AI que desarrollarán AR. Nosotros desarrollamos una regla de predicción, utilizando datos del "Leiden Early Artritis Clinic", una cohorte de pacientes con artritis de reciente comienzo (n= 1700). Se seleccionaron los pacientes que se presentaron con AI (n= 570); la progresión a AR u otros diagnósticos fue monitoreada después de un año de seguimiento. La regla de predicción consistió en nueve variables clínicas: género, edad, localización de los síntomas, rigidez matinal, conteo de articulaciones inflamadas y dolorosas, proteína C reactiva, factor reumatoide, y anticuerpos antipéptido citrulinado cíclico (antiCCP). Cada conteo de predicción varia entre 0 y 14 y corresponde a una probabilidad (porcentaje) de desarrollar AR. De este modo, en AI temprana, el riesgo de desarrollar AR puede predecirse, permitiendo individualizar las decisiones terapéuticas para iniciar medicamentos modificadores de la enfermedad en pacientes que se presenten con AI.
Palabras clave: artritis reumatoide, artritis indiferenciada, predicción, prevención.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that may have a high impact on patients' quality of life as it is associated with disability, (co)morbidity and an increased mortality rate1. The last decade it has been recognized that RA needs to be diagnosed early and treated promptly with disease modifying antirheumatic drugs in order to successfully interfere with the disease process. This new treatment paradigm in combination with new treatment options have already improved the prospects for RA patients in general and have lead to a reduction in the level of joint destruction, disability and mortality. Although rheumatologist are nowadays successful in reducing the level of disease activity patients in with RA, the ultimate challenge for the future is to initiate therapy in such an early phase that the actual development of RA is prevented. This indicates that patients should be treated in a phase that they have not fully developed the disease. It might well be that in such an early phase the mechanisms that drive chronicity are less settled and that interference with the disease process will induce remission more easily. To achieve this rheumatologists require two tools. First, they should be able to identify the patients that will develop RA and, second, drugs that are proven to be effective in preventing the development of RA should be available.
Currently clinical trial are designed in order to assess treatment efficacy in patients with early undifferentiated arthritis (UA), this manuscript appraises the definition of UA, the natural course of UA, clinical characteristics that predict the progression from UA to RA and pathophysiological differences between UA and RA. Finally, results on the first trial investigating the effect of DMARD therapy in patients with UA are presented.
Definitions of early arthritis and UA
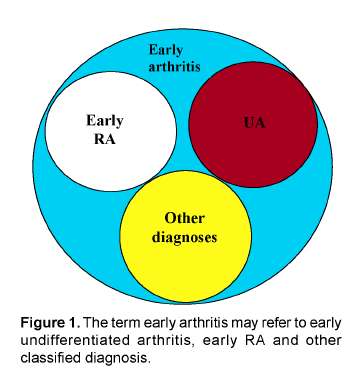
The published trials evaluating treatment strategies in RA all include patients classified according to the 1987 ACRcriteria for RA. These criteria are generally accepted and are developed by experts that compared characteristics of patients with longstanding "classical" RA (mean disease duration 8 years). In clinical practice, patients presenting with an early arthritis frequently have an undifferentiated disease that in time may progress to a polyarthritis fulfilling the ACRcriteria for RA or may have a more benign disease course. The ACR criteria have been criticized as they have low discriminative ability in patients presenting with recent onset arthritis25. This is not surprising considering the method by which the criteria were formulated and the components of the ACRcriteria. One of the criteria is the presence of erosions on the radiographs of hands and wrists. In the early phases of RA only 13% of the patients have erosive disease6. Additionally, erosions often initially present in the small joints of the feet and appear in the small joints of the hands at a later point in the disease course7. Also rheumatoid nodules are very rare in the early phases of RA and rheumatoid factor is present in only 50% of the patients with early RA8. This indicates that at present a set of criteria is needed that applies to early undifferentiated arthritis and that differentiate the UApatients that will progress to RA from those that will have a more benign disease course. Before the characteristics that predict the disease outcome in UApatients can be identified, a general acceptance on the definition for early UA is needed. In the literature several terms that refer to arthritis of recent onset are used, but they refer to distinct categories of patients and should therefore be separated. Most frequently used are the terms "early arthritis", "early R A" and undifferentiated arthritis. Early arthritis is the description of a state in which there is a (mono, oligo or poly) arthritis that has a recent onset. In case of early arthritis the disease can be undifferentiated or differentiated (Figure 1). For example, about 20% of the patients that present with an early arthritis directly fulfill the ACRcriteria and thus can be classified as RA. This indicates that in early RA per definition the ACRcriteria for RA are fulfilled. Since the ACR criteria also state that the patients fulfill the criteria for at least 6 weeks, shorter disease duration than six weeks is by definition impossible in case of early RA. Patients with an early arthritis may also fulfill classification criteria for other diagnoses. Finally, those early arthritis patients that can not be classified according to ACRcriteria and in whom the arthritis is not septic or reactive in origin have per exclusionem an undifferentiated arthritis. Discerning UA from early arthritis and early RA is relevant when comparing studies that describe models that predict the disease outcome or studies that assess therapeutic efficacy as the generalizability of these studies depends on the patient group that is included. This manuscript focuses on UA as patients with UA may advance to RA. Therefore, these patients may provide an opportunity as it is to be expected that the process that drives chronicity can be influenced more effectively when it is less established.
Natural disease course of UA
The natural disease course of UA is variably reported in several inception cohorts. This is not only due to the use of different definitions for UA, but is also a result of differences in inclusion criteria for several early arthritis cohorts. For example, inclusion in the Norfolk Arthritis Registry (UK) required the presence of at least two swollen joints9, whereas for inclusion in the Leeds early arthritis clinic (UK)10 or the arthritis cohort from Wichita (USA) the presence of synovitis was not required11. On the other hand, some early arthritis clinics did not include patients with UA but only patients that fulfilled the criteria for RA12,13. Inclusion criteria from early arthritis cohorts differ not only in the presence/absence of arthritis, but also in the required symptom duration. Patients could be included in the NOAR when the arthritis was present for at least 4 weeks, whereas a symptom duration of more than 12 weeks was an exclusion criteria for the early arthritis cohort from Birmingham. Different inclusion and exclusion criteria instigate the enrolment of different groups of patients and clarifies that different results are observed when the natural disease course is studied.
Early arthritis cohorts that included all patients with at least one swollen joint reported that at initial presentation about 20% of the patients fulfilled the criteria for RA and that 35%54% of the patients presented with UA14,15. In case of UA the disease course was divers: 4055% remitted spontaneously1518, 35%50%6,14 developed RA and the remaining patients developed other diagnoses or remained undifferentiated (Figure 2).
These data also illustrate that when evaluating studies on UApatients the duration of symptoms are of importance for the outcome of the patient group. In other words, an undifferentiated arthritis from recent onset (several weeks) has a different natural course than an arthritis that after one year of followup is still unclassified (persistent undifferentiated arthritis). In the Leiden Early Arthritis Clinic, patients that after 1 year of followup had persistent undifferentiated arthritis developed only in a minority (10%) RA later on in the disease course.
Intriguingly, the reported rates of spontaneous remission patients in case of UA are importantly different from those in RA. Whereas remission was achieved in 4055% of the patients with recentonset undifferentiated arthritis, the remission rate in RA is at most 1015%19, 20. Apparently, the chance to achieve a natural remission becomes smaller when the disease process is more matured. This supports the notion that chronicity might be more easily reversed in the phase of UA.
Predicting progression from UA to RA
As UA has a variable disease course and DMARDtherapy is potentially toxic, only the UApatients that have a high chance to develop RA are preferentially treated with DMARDs, whereas the patients that will achieve a spontaneous remission will preferentially not receive these drugs. This underlines that a model that is able to predict the disease outcome in individual patients with UA is needed. Initial attempts to define such prognostic criteria have been made by Visser et al. based on the Leiden Early Arthritis Cohort21. This model predicts disease persistency and erosiveness. For the development of this model all early arthritis patients were included and not only patients with UA. Consequently, patients that at first presentation were classified as e.g. reactive arthritis or RA were also included. However, the natural course of these diseases is already known as reactive arthritis is in most cases remitting and RA is in most cases a persistent
disorder, indicating that patients with a diagnosis of which the disease course is wellknown may skew a model that predicts the disease outcome. As the model of Visser et al. was not developed using specifically patients with UA, this model is not optimal to guide individualized treatment decision in UA. Recently, a model that predicts the disease outcome in individual patients with UA was developed, also based on the Leiden Early Arthritis Cohort22. From a total cohort of 1700 early arthritis patients, 570 patients presented with UA. After one year of followup 31% of the UApatients had progressed to RA. The remaining twothird had developed other diagnoses (16%), had achieved spontaneous remission (26%) or remained unclassified (26%). Clinical characteristics between the UApatients that had and had not developed RA were compared and using logistic regression analysis the variables that were independent predictors for the development of RA were selected. This resulted in the construction of a prediction rule (Figure 3)22. The discriminative ability of this prediction rule was assessed by the area under the receiver operator curve, which was 0.89 for the derivation cohort and 0.97 for the replication cohort. The total prediction score ranged between 0 and 14. All patients with a score < 4 did not progress and all patients with a score > 10 did progress to RA. With the cutoff levels < 6 and > 8 the negative and positive predictive values were 91% and 84% respectively. As this prediction rule consists of 9 variables that are regularly assessed at the outpatient clinic (age, gender, distribution of involved joints, morning stiffness severity, number of tender and swollen joints, Creactive proteins, rheumatoid factor and antiCCP antibodies), this prediction rule can be easily applied in daily practice. Moreover, as the prediction rule estimates the chance for an individual patient to progress to RA in a percentage, application of this rule might facilitate the involvement of patients themselves in treatments decisionmaking.
Biological mechanisms in UA and RA
Subsequently, the question arises which biological mechanism are responsible for the progression from UA to RA. The identified nine risk factors may provide clues. Therefore possible mechanisms underlying the association with each of these variables and RAdevelopment are shortly discussed.
1. Age
Ageing is associated with a decline in a large number of physiological functions as well as immune function. Impairment in cellular, humoral and innate immunity might predispose persons with an increasing age to amongst others RA. Relevant changes in the innate immune system are an altered phagocyte function and an increased production of proinflammatory cytokines such as Il1, TNFalpha and IL6 (the latter is responsible for the increase in CRP that is seen in elderly persons)23. Modification of the adaptive immune system is exemplified by the development of a polyreactive antibody production at higher age24. The immunosenescence is further discussed in reference 25 but might predispose to arthritis or mediate an aggressive disease course.
2. Gender
Sex hormones influence the predisposition to autoimmune diseases. In general, men are less prone than women. This might be caused by antiinflammatory effects of androgens. Recently it was demonstrated that PPARá, a gene in CD4+ T cells, is sensitive to androgen levels and is higher expressed in males, which induced higher levels of Th2 cytokines and consequently a lower susceptibility to Th1mediated autoimmune diseases26. Estrogen are also able to suppress arthritis in mouse models27 and the use of oral anticontraceptives might be associated with a lower risk on RAdevelopment28. However, this finding was not replicated in the Nurses Health study29. Additionally, both estrogen and androgen inhibit bone resorption30. Moreover, sex hormones may have local effects which seems to consist mainly in modulation of cell proliferation and cytokine production (i.e., TNFalpha, IL1). Altogether, these data suggest that postmenopausal women exhibit a proinflammatory cytokine profile, that might contribute to the higher incidence of RA in these women.
3. Distribution of involved joints
RA particularly affects the small joints of the hands and feet, whereas in some other rheumatologic diseases the large joints are preferentially inflamed. At present the reason for this predilection is not clear. It has recently been suggested that differential accumulation of regularly T cells in different joints may dictate the anatomic spectrum seen in arthritis syndromes31. However this hypothesis is based on animal models and whether this might explain the distribution of inflamed joints in human is not known.
4. Severity of morning stiffness
Although in clinical practice the presence of morning stiffness is a specific maker for RA, the anatomical substrate causing morning stiffness is ample examined. Straub et al. recently proposed that the symptom stiffness is due to edema formation mediated by circulating proinflammatory cytokines32. The observations that the proinflammatory cytokines TNFα and Il6 exhibit a circadian rhythm and that these levels peak level around 6.00 7.00 in RApatients might support this hypothesis and explain why stiffness is most severe in the early morning.
5. Creactive protein, number of tender and swollen joints
As already discussed IL6 enhances the hepatic production of CRP, explaining that in situations in which IL6 is increased (older age, inflammation) CRPlevels are elevated. Therefore, the CRPlevel directly reflects the level of proinflammatory cytokines. Additionally, also the number of tender joints and the number of swollen joint may mirror the level of the proinflammatory processes. It is reasonable to suggest that in case of increased (local) proinflammatory activity the biological processes that generate RA are boosted.
6. Rheumatoid factor and antiCCP antibodies
The association between most of the above mentioned factors and RA are (in part) mediated by an increase in proinflammatory cytokines, and thus reflect a quantitative trait. The last two items of the prediction model, the presence of autoantibodies, are primarily a qualitative trait. Although it is still uncertain whether these autoantibodies are of pathophysiological importance or the result of a bystander effect, the specificity of antiCCP antibodies for the development of RA is extensively reported. A recent study revealed that not only the presence of antiCCP antibodies, but in case of antiCCPpositivity also the level of these antibodies, is correlated with an increased risk on progressing from UA to RA33. Moreover, not only the level but also the nature of the autoantibody response is different in UA and RA. Patients with UA have a lower number of antiCCP isotypes than patients with RA and, similarly, the UApatients that progressed to RA had a higher number of isotypes compared to the UApatients that did not develop RA34.
In conclusion, the biological mechanisms underlying UA and RA differ both in quantity (e.g. level proinflammatory cytokines) and quality (e.g. autoantibody response). Apparently, UApatients that have more of these quantitative or qualitative traits have a concomitant higher risk to progress to RA.
Outcomes of treatment in UA
Almost all clinical trials on therapeutic strategies have included patients with (early of longstanding) RA. At present there is one study that assessed the efficacy of methotrexate in patients with UA35. In this double blind clinical trial patients were randomized for treatment with either methotrexate or placebo. The aim of the PROMPT study was to determine whether patients with UA benefit fromtreatment with methotrexate (MTX). The main outcomes were progression to RA and radiographic joint damage.
The PROMPT study was a prospective doubleblind placebocontrolled randomized multicenter trial in 110 patients with UA who fulfilled the ACR 1958 criteria for probable RA. Treatment started with MTX 15 mg/wk or placebo tablets, and dose increase was dictated by 3monthly calculations of the disease activity score (DAS), aiming at a DAS= 2.4. After 12 months, the study medication was tapered to nil. Patients were followed up for 30 months. When a patient fulfilled the ACR 1987 criteria for RA, the study medication was changed to MTX.
In the MTXgroup, 22/55 patients had progressed to RA versus 29/55 in the placebogroup, the criteria were fulfilled at a later time point (p=0.04), and patients showed less radiographic progression over 18 months (p=0.046). Subsequently, patients were followed for 30 months and both the progression towards RA and the level of joint destruction were measured. A significant lower number of UApatients that was treated with methotrexate had progressed to RA compared to the placebo treated patients. In addition, the UApatients that were treated with methotrexate had a significantly lower lever of radiological joint destruction, indicating a less severe disease course. Interestingly, after the cessation of methotrexate at 18 months the difference in the number of patients that developed RA remained statistically significant but the difference became smaller. This suggests that in some patients methotrexate had hampered the progression of the disease but had not been able to totally stop the underlying pathophysiological mechanisms. These data have to be replicated in other studies and hopefully future targeted therapies will be able to fully halt the development of persistent arthritis. Nevertheless, the data of this study is promising as they indicate that treatment in an early phase of RA, before the disease is established, is effective.
Conclusion
UA is a diagnosis per exclusionem and refers to arthritis that cannot be classified according to current criteria. The term UA is different from "early arthritis" and "early RA". The disease course of UA is variable and about one third of the UApatients are in an early phase of RA. These UApatients provide an opportunity, as the disease process in UA is less established and treatment in this early phase might result in halting the progression towards RA. To achieve this, physicians should be able to predict which UApatients will progress to RA and will benefit from drugs that are proven to be effective in UA. A rule that predicts the chance to develop RA in individual patients with UA has recently been developed and clinical triáis evaluating the effects of DMARDtherapy in UA are being designed. Hopefully, in the next decade personalized medicine will be achieved and the impact of arthritis on patients' quality of life will be further diminished.
Parts of this manuscript have been published before, for an extensive literature search see papers published by prof. Dr. TWJ Huizinga via www.pubmed.com
References
1. Pincus T, Callahan LF. What is the natural history of rheumatoid arthritis?. Rheum Dis Clin North Am 1993; 19(1): 123-151. [ Links ]
2. Harrison BJ, Symmons DP, Barrett EM, Silman AJ. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis. American Rheumatism Association. J Rheumatol 1998; 25(12): 2324-2330. [ Links ]
3. Harrison B, Symmons D. Early inflammatory polyarthritis: results from the Norfolk Arthritis Register with a review of the literature. II. Outcome at three years. Rheumatology (Oxford) 2000; 39(9): 939-949. [ Links ]
4. Symmons DP, Hazes JM, Silman AJ. Cases of early inflammatory polyarthritis should not be classified as having rheumatoid arthritis. J Rheumatol 2003; 30(5): 902-904. [ Links ]
5. Green M, MarzoOrtega H, McGonagle D, et al. Persistence of mild, early inflammatory arthritis: The importance of disease duration, rheumatoid factor, and the shared epitope. Arthritis Rheum 1999; 42: 2184-2188. [ Links ]
6. Machold KP, Stamm TA, Eberl GJ, Nell VK, Dunky A, Uffmann M, Smolen JS. Very recent onset arthritisclinical, laboratory, and radiological findings during the first year of disease. J Rheumatol 2002; 29(11): 2278-2287. [ Links ]
7. van der Heijde DM, van Leeuwen MA, van Riel PL, van de Putte LB. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharps method (van der Heijde modification). J Rheumatol 1995; 22(9): 1792-1796. [ Links ]
8. Nell VP, Machold KP, Stamm TA, Eberl G, Heinzl H, Uffmann M, Smolen JS, Steiner G. Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis 2005; 64(12): 1731-1736. [ Links ]
9. Symmons DP, Silman AJ. The Norfolk Arthritis Register (NOAR). Clin Exp Rheumatol 2003; 21(5 Suppl 31): S94-S99. [ Links ]
10. Quinn MA, Green MJ, MarzoOrtega H, Proudman S, Karim Z, Wakefield RJ, Conaghan PG, Emery P. Prognostic factors in a large cohort of patients with early undifferentiated inflammatory arthritis after application of a structured management protocol. Arthritis Rheum 2003; 48(11): 3039-3045. [ Links ]
11. Wolfe F, Ross K, Hawley DJ, Roberts FK, Cathey MA. The prognosis of rheumatoid arthritis and undifferentiated polyarthritis syndrome in the clinic: a study of 1141 patients. J Rheumatol 1993; 20(12): 2005-2009. [ Links ]
12. Sokka T. Early rheumatoid arthritis in Finland. Clin Exp Rheumatol 2003; 21(5 Suppl 31): S133-S137. [ Links ]
13. Kvien TK, Uhlig T. The Oslo experience with arthritis registries. Clin Exp Rheumatol 2003; 21(5 Suppl 31): S118-S122. [ Links ]
14. van Aken J, van Bilsen JH, Allaart CF, Huizinga TW, Breedveld FC. The Leiden Early Arthritis Clinic. Clin Exp Rheumatol 2003; 21(5 Suppl 31): S100-S105. [ Links ]
15. Hulsemann JL, Zeidler H. Undifferentiated arthritis in an early synovitis outpatient clinic. Clin Exp Rheumatol 1995; 13(1): 37-43. [ Links ]
16. van Aken J, Van Dongen H, le Cessie S, Allaart CF, Breedveld FC, Huizinga TW. Longterm outcome of rheumatoid arthritis that presented with undifferentiated arthritis compared to rheumatoid arthritis at presentation an observational cohort study. Ann Rheum Dis 2006; 65(1): 20-25. [ Links ]
17. Tunn EJ, Bacon PA. Differentiating persistent from selflimiting symmetrical synovitis in an early arthritis clinic. Br J Rheumatol 1993; 32(2): 97-103. [ Links ]
18. Harrison BJ, Symmons DP, Brennan P, Barrett EM, Silman AJ. Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol 1996; 35(11): 1096-1100. [ Links ]
19. LinnRasker S P, Allaart CF, Kloppenburg M, Breedveld FC, Huizinga TWJ. Sustained remission in a cohort of patients with RA: association with absence of IgMrheumatoid factor and absence of antiCCP antibodies. Int J Advances Rheumatology 2004; 2(4): 46. [ Links ]
20. van der Helmvan Mil AH, Dieude P, Schonkeren JJ, Cornelis F, Huizinga TW. No association between tumour necrosis factor receptor type 2 gene polymorphism and rheumatoid arthritis severity: a comparison of the extremes of phenotypes. Rheumatology (Oxford) 2004; 43(10): 1232-1234. [ Links ]
21. Visser H, le Cessie S, Vo s K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 2002; 46(2): 357-365. [ Links ]
22. Van der Helmvan Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes REM, Huizinga TWJ. A rule to predict disease outcome in patients with recentonset undifferentiated arthritis to guide individual treatment decisions. Arthritis Rheum. 2007; 56(2): 433-440. [ Links ]
23. Mishto M, Santoro A, Bellavista E, Bonafe M, Monti D, Franceschi C. Immunoproteasomes and immunosenescence. Ageing Res Rev 2003; 2(4): 419-432. [ Links ]
24. Johnson SA, Cambier JC. Ageing, autoimmunity and arthritis: senescence of the B cell compartment implications for humoral immunity. Arthritis Res Ther 2004; 6(4): 131-139. [ Links ]
25. Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes Infect 2001; 3(10): 851-857. [ Links ]
26. Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, et al. Peroxisome proliferatoractivated receptor (PPAR){alpha} expression in T cells mediates gender differences in development of T cellmediated autoimmunity. J Exp Med. 2007; 204(2): 321-330. [ Links ]
27. Yoneda T, Ishimaru N, Arakaki R, Kobayashi M, Izawa T, Moriyama K, Hayashi Y. Estrogen deficiency accelerates murine autoimmune arthritis associated with receptor activator of nuclear factorkappa B ligandmediated osteoclastogenesis. Endocrinology 2004; 145(5): 2384-2391. [ Links ]
28. Doran MF, Crowson CS, OFallon WM, Gabriel SE. The effect of oral contraceptives and estrogen replacement therapy on the risk of rheumatoid arthritis: a population based study. J Rheumatol 2004; 31(2): 207-213. [ Links ]
29. Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breastfeeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses Health Study. Arthritis Rheum 2004; 50(11): 3458-3467. [ Links ]
30. Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 2005; 328(3): 688-696. [ Links ]
31. Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum 2007; 56(2): 509-520. [ Links ]
32. Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis Rheum 2007; 56(2): 399-408. [ Links ]
33. van der Helmvan Mil AH, Verpoort KN, le Cessie S, Huizinga TW, de Vries RR, Toes RE. The HLADRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum 2007; 56(2): 425-432. [ Links ]
34. Verpoort KN, Jolvan der Zijde CM, Papendrechtvan der Voort EA, IoanFacsinay A, Drijfhout JW, van Tol MJ, Breedveld FC, Huizinga TW, Toes RE. Isotype distribution of anticyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum 2006; 54(12): 3799-3808. [ Links ]
35. van Dongen H, van Aken J, Lard LR, et al. Treatment of Patients with Undifferentiated Arthritis with Methotrexate: a DoubleBlind, PlaceboControlled, Randomized Clinical Trial to Prevent Evolvement into RA. ACR 2005, abstract (479)L4. [ Links ]