Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Reumatología
versión impresa ISSN 0121-8123
Rev.Colomb.Reumatol. vol.23 no.2 Bogotá abr./jun 2016
Case report
Pulmonary alveolar microlithiasis*
Microlitiasis alveolar pulmonar
Jesús Giovanny Ballesteros Muñoza,b,**, Jorge Enrique Medina Rosasb,c, Juan Manuel Bello Gualterod, John Darío Londoño Patiñob,c, Claudia Marsella Guzmán Vergarad, César Augusto Gutiérreze, José Ignacio Angarita Céspedese, Rafael Valle-Oñatea,d
a Departamento de Reumatología, Facultad de Medicina, Universidad Militar Nueva Granada, Bogotá, D.C., Colombia
b Grupo de Estudio de Espondiloartropatías, Facultad de Medicina, Universidad de La Sabana, Bogotá, D.C., Colombia
c Departamento de Reumatología, Facultad de Medicina, Universidad de La Sabana, Bogotá, D.C., Colombia
d Departamento de Reumatología, Facultad de Medicina, Hospital Militar Central, Bogotá, D.C., Colombia
e Hospital La Victoria, Universidad de La Sabana, Bogotá, D.C., Colombia
* Please cite this article as: Ballesteros Munoz JG, Medina Rosas JE, Bello Gualtero JM, Londono Patino JD, Marsella Guzmán Vergara C, Gutiérrez CA, et al. Microlitiasis alveolar pulmonar. Rev Colomb Reumatol. 2016;23:115-120.
** Corresponding author. E-mail address: jgballesterosmunoz@gmail.com (J.G. Ballesteros Munoz).
Article history: Received 13 October 2013 Accepted 24 February 2016 Available online 8 August 2016
Abstract
Pulmonary alveolar microlithiasis is an uncommon disease of unknown etiology, and is characterized by the presence of multiple sub-pleural and intra-alveolar microcalcifications. We present the case of a patient with rheumatoid arthritis and chronic renal disease, but with no respiratory symptoms.
Keyword: Pulmonary alveolar microlithiasis.
Resumen
La microlitiasis pulmonar alveolar es una enfermedad infrecuente, de etiología desconocida, caracterizada por la presencia de múltiples microcalcificaciones intraalveolares y subpleurales. Presentamos el caso de un paciente asintomático respiratorio, con historia clínica de artritis reumatoide y enfermedad renal crónica.
Palabras clave: Microlitiasis alveolar pulmonar.
Introduction
Pulmonary alveolar microlithiasis (PAM) is a rare entity characterized by the presence of microscopic granules of calcium phosphate at the alveolar level, known as microliths or calcospherites.1-4 Epidemiologically, the number of cases described in the world varies according to the bibliographic references, although not exceeding 600 cases,1 with no specific geographic predilection, being reported cases in all continents.5-8 It is most frequently observed in Europe and Asia, being Japan,8 Turkey9,10 and Italy11,12 the countries with the largest number of cases.
It is considered a systemic disease13 and two forms of presentation have been described, the first is of familial character in up to 50% of cases14 and the second is of sporadic character, having no direct relationship with the family members.15
The incidence according to the gender is variable, although in some case series is reported to be 2:1, female: male, with a predominance of familial occurrence,16 other series make reference to a higher proportion in men, being most of sporadic occurrence, as in the cases in which chest X-rays are taken routinely to meet job requirements or to join the army.8,12 The age of presentation is not defined, since cases are reported in all ages,16 most of them being described between the second and fifth decades of life.5,8,17
In Colombia are known 6 cases which can be tracked and referenced by different databases (including this one), with a predominantly familial presentation.18-21
Case presentation
It is a 44-year-old man, who consulted the Emergency Department of the hospital because a clinical picture of 6 days of evolution, consisting of heartburn epigastric pain, associated with nausea and non-quantified fever. He reported a history of rheumatoid arthritis diagnosed 15 years earlier, right nephrectomy due to pyelonephritis, chronic use of nonsteroidal anti-inflammatory drugs, and secondary chronic kidney disease; he denied that he was taking glucocorticoids; and within the family medical history he manifested the presence of lung disease in a brother (later specified as PAM). Smoking of 3.5 packages/year. He referred not having had respiratory symptoms such as cough of dyspnea, and neither the presence of pollakiuria. He denied having joint inflammation or morning stiffness, and occasionally he had pain in the second metacarpophalangeal joint of the right hand.
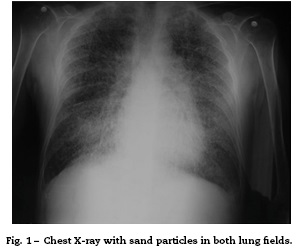
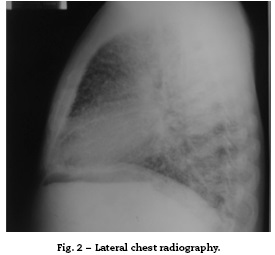
On admission the patient was afebrile, dehydrated, with the following vital signs: blood pressure 120/75 mmHg, heart rate 90 beats per minute, respiratory rate 18 breaths per minute, weight 47.5 kg, height 1.62 m, body mass index 18.1 kg/m2. Arterial oxygen saturation of 91%, with an inspired fraction of oxygen of 0.21. On the general physical examination, added lung sounds were not found at pulmonary auscultation; abdomen soft, with no signs of peritoneal irritation. There was not found joint swelling or pain, hands with swan-neck deformities and ulnar deviation. No other remarkable alterations. Normal electrocardiograma The hemogram showed anemia with normal corpuscular volumes, discrete leukocytosis at the expense of neutrophils and mild thrombocytosis. The blood urea nitrogen and the serum creatinine were elevated, urinalysis with presence of leukocyturia, hematuria, and positive nitrites in addition to granular casts. The chest radiography revealed the presence of diffuse micronodular opacities in both lung fields, and numerous calcifications of the size of "grains of sand" were observed on the high resolution computed tomography of the chest.
Miliary tuberculosis was considered as a diagnostic probability, and therefore, antituberculous treatment was started; the patient evolves without changes in his condition, improvement in urinary symptoms with the initiation of parenteral antibiotic therapy and improvement of the abdominal pain, without deterioration of his respiratory pattern. The patient is assessed by the service of Pulmonology, which considers that due to the findings in the chest radiography and tomography, it is appropriate to continue the antituberculous therapy and schedule a fibrobronchoscopy. Subsequently was assessed by the Nephrology service, which considered to follow-up the patient for possible initiation of hemodialysis.
Other studies showed a rheumatoid factor of 495 U/ml by nephelometry; levels of folic acid, serum iron, transferrin, intact parathyroid hormone, serology, ELISA HIV, and serology for hepatitis B and C were reported as negative and normal. The transthoracic echocardiogram showed a preserved ejection fraction of 65%, mild diastolic dysfunction and mild pulmonary hypertension.
It was performed a fibrobronchoscopy, in which normal lobar and segmental divisions were found, a bronchoalveolar lavage of the left anterior basal segment was carried out, and a transbronchial biopsy was taken; the result of the broncho-alveolar lavage showed scarce polymorphonuclear leukocytes per field, the direct examination with potassium hydroxide and the Ziehl-Neelsen stain were negative, the determination of adenosine deaminase and the cultures were also negative. The patient evolves favorably without dyspnea, with resolution of the abdominal pain, and with adequate tolerance of the antituberculous treatment, and it was decided to discharge him with an order to continue the antituberculous therapy on an outpatient basis and to attend the outpatient clinic for a control with the pathology report. The patient was assessed in the outpatient clinic of Pulmonology, were the medical history was expanded with his family members, mentioning that the brother was diagnosed with PAM 4 years earlier, with a pathology report suggesting the presence of PAM, due to the finding of spherical and lamellar calcifications, without identifying granulomas or malignancies in the revised material. For this reason it was decided to discontinue the antituberculous therapy and to continue periodical controls by Pulmonology and Nephrology. The biopsy was reviewed by the Service of Pathology of the Simon Bolívar University Hospital of third level, as well as by the National Institute of Health (Instituto Nacional de Salud) confirming the diagnosis of PAM.
Discussion
The PAM was first described macroscopically by Malpighi in 1686.2 Subsequently, in 1856, Friederich is who describes several forms of amylaceous bodies in the lung, which suggested
to be within the alveoli, similar to those of the prostate.3,22 In 1918, Harbitz provides the histopathological findings and Schildknecht (1932) the radiological findings.1 But Ludwig Puhr is who in 1933, coins the term mikrolithiasis alveolaris pulmonum,3,13,23 as it is currently known.
In 1947 Mariani et al., are the first to make a complete description of the disease, from the clinical, functional and radiological viewpoints,1,2 without making any mention of the infectious component.14,24
It has been observed a hereditary component, predom-inantly an autosomal recessive disorder,5 being recognized the mutation in the SLC34A2 gene that encodes a NaPi-II cotransporter; this transporter is a membrane protein which is expressed predominantly in the lungs and the mammary glands of the mammals, less recognized in the bowel, the kidney and the prostate, being the unique phosphate carrier, expressed in the type II pneumocytes. It is well known that when the pulmonary surfactant is degraded or recycled, phosphates that are product of the metabolism of phospholipids remain in the alveolar space, and when the mutation on the carrier protein is present, the elimination of these phosphates can be reduced, with the consequent formation of microliths.25-29
Within its etiology, it has been attempted to associate it with expositional components,18,30,31 specifically with the consumption of snuff in Thailand, which is a mixture of dry tobacco, oriental gum and artisanal ingredients containing high concentrations of calcium carbonates and phosphates.32
At the time of diagnosis more than half of the patients are asymptomatic and the pulmonary findings are fortuitously.33 The course of the disease is slow and progressive. As the disease progresses, the symptoms begin, mainly the dyspnea.12 The cough does not appear to be a common complaint in the majority of the patients, although in some of them it may be persistent.34 In addition, asthenia, chest pain, palpitations and weight loss are described,35 occasionally, cyanosis and digital clubbing are the first clinical signs.1 Expulsion of microcalculi and hemoptysis have been also described.20,36 Since most of the literature is of case reports, the normal course of the disease is unknown, assuming a chronic course, without fulminant presentations, but with rapid progressions, especially in children; the most common in the majority of cases is that there is a poor correlation between the symptoms and the radiological findings.12,37 Although these complications are rare, within the spectrum of the disease are described other organs involved,8 among which are the testicles (testicular microlithiasis) with subsequent azoospermia, in addition to commitment of the sympathetic nervous system38,39 and nephrocalcinosis.40,41 Other sites even less frequent are the epididymis, the seminal vesicles and the prostate.2,42 There are single reports of commitment of the pericardium and hypertrophic osteoarthropathy.2,43 No effective medical therapy is currently known, in the majority of cases the disease follows its natural course and it is considered that the patient can be asymptomatic for decades, the progression of the dis-ease after diagnosis is slow. Without exception, all untreated patients have fatal outcomes secondary to cardiac or respiratory failure.1
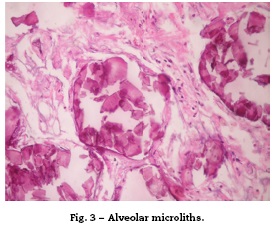
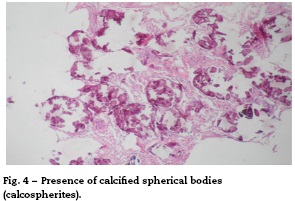
There are different means to make the diagnosis; the simplest is the direct study of the sputum, specifically looking for microliths, although its diagnostic performance is limited (Figs. 1-4).1,3
The majority of tests (creatinine, urea nitrogen, glycemia, urinalysis) may be found normal, polyglobulia is occasionally found in the blood count.5,8 The serum levels of calcium, magnesium and intact parathyroid hormone are normal,17 although there have been described patients with hypercalcemia (a rare situation). Elevations of the serum levels of the pulmonary surfactant proteins A and D have been reported recently, being considered that they could become a tool for the follow up of the patients.8
Among the diagnostic tests, it must be done first a chest X-ray, which usually shows bilateral micronodular calcifications, linear reticular opacities and uniformity in the size of the microliths distributed throughout the bronchial tree. The above findings have been described as "sandstorm"44 or "snowstorm",45 which predominate in the basal and medial areas of the lung, with obliteration of the cardiac and diaphragmatic edges.35
In the subpleural surfaces are seen deposits, resulting in a linear opacity that demarcates the pulmonary and mediastinal divisions,46 known as black pleural line, which was described by Felson, and is considered as a visual illusion secondary to a radiolucent area between the pleural parenchyma and the ribs.45,47-50 Other typical findings are small apical bullae and honeycomb images, without mention of lymph node involvement.51
The findings on the chest tomography show bilateral intra-alveolar calcified nodules,20,35 there also can be seen areas of high attenuation in ground glass which seem to correspond to small deposits of microliths (smaller than one millimeter), with a predilection for the bases and the posterior segments of the lungs.52 When these areas of ground glass are associated with thickening of the septa, they are known as "crazypaving" pattern, which is an infrequent presentation.47,51 Other findings observed in advanced stages of the disease are the presence of spontaneous pneumothorax,53 and less frequently calcified nodules, subchondral air cysts and confluent nodules that may eventually form consolidations in the air space.20,54
When the pulmonary scintigraphy with technetium 99 is used, it helps to confirm the calcic nature of the pulmonary lesions, with intense diffuse uptake of the radioisotope at the pulmonary level. It should be clarified that in the early stages of the disease, a significant uptake of the technetium 99 might not be found.47,55
When PAM is suspected in cases of sporadic presentation and having abnormal diagnostic images, is necessary the use of other diagnostic tests, such as bronchoscopy and broncho-alveolar lavage,56,57 with which the presence of microliths can be demonstrated, complemented with a biopsy obtained by bronchoscopy, especially in pediatric patients. Today is very rare the use of an open biopsy, although in some cases it is necessary.3,58,59
Regarding the histopathological findings, there are numerous laminated calcospherites, scarce fibrosis of the alveolar walls and presence of hemosiderinladen macrophages. Being this the report of a large number of cases. The lung parenchyma, as the disease progresses, degenerates causing bullae and interstitial fibrosis. Some cases have been described in which the calcospherites involve the septal walls, not only at the level of the alveolar space, but also at the bronchial level.3,22
In those cases in which there is a family history of PAM, the diagnosis is established with the clinical picture and the imaging findings, either lung radiography or tomography showing pulmonary infiltrates with sandstorm pattern, micronodular calcifications along the bronchovascular bundles, thickening of the interlobular septa, involving predominantly and symmetrically the lower lobes, without having to subject the patient to invasive tests such as fibrobronchoscopy and bronchoalveolar lavage.
When the microliths are analyzed, they look irregular shaped, rounded and occasionally oval, composed of laminae with measures that may range between 0.01 and 0.3 mm,55 which appear to increase in size with age until they occupy all the alveolar space60 and in the advanced stages they come into contact with the walls, which become damaged and then are replaced by fibrous tissue. The microliths are compounds of calcium, phosphorus, and low concentrations of iron, zinc, aluminum and magnesium.1,22,61 They are located at first in the lower lobes, but over time (20-30 years), they extend to the whole lung.
The majority of the patients reported in the literature exhibit abnormalities in the pulmonary function tests, prevailing the reduction of the forced expiratory volume in the first second and the vital capacity, in addition to decreased functional residual capacity, residual volume and total lung capacity.3,5,62
PAM has been found to be related to other diseases such as tuberculosis (sometimes it is the first suspected diagnosis), lymphocytic interstitial pneumonitis, pneumonoconiosis and mitral stenosis.2
There are publications in which different therapeutic measures have been suggested with the view to removing the intraalveolar calcospherites. The first measure that was suggested was the use of bronchoalveolar lavage, which did not reported clinical improvement or significant benefits for the patients, despite the fact that it was observed that the fluid obtained had a sandy appearance.3,24,60 Another measure has been the use of chelating agents and glucocorticosteroids, which also delivered unsatisfactory results.63 In advanced stages, as a measure of symptomatic management it has been proposed the continuous use of oxygen, with positive pressure, with paradoxical results. It has been described the use of sodium etidronate, a compound known by inhibiting the formation of hydroxyapatite, with improvement of the pulmonary infiltrates,1,3,64 however, none of these measures has shown results that are strong enough to be established as a definitive treatment. There is a case report of 2 patients diagnosed at 5 and 9 years of age who received treatment with etidronate for 9 and 11 years, respectively, in whom clinical and radiological improvement was observed. There are not clear indications for lung transplantation due to the lack of prognostic indices and to the insidious nature of the disease, however, it is the only effective measure and should be considered when there is right heart failure or severe respiratory failure; to maximize the chances of success, the patients should be referred before they present severe right ventricular dysfunction.1,3,64
This reported case makes reference to a case of PAM, diagnosed by diagnostic imaging and anatomopathological studies, which were reviewed by several specialists in the areas of Pulmonology and Pathology of the city of Bogota.
This diagnosis was clarified, given that the brother of the patient presented an entity which initially was unknown by the service, but which it had been established several years ago as PAM, after a routine medical checkup. An attempt was made to access the clinical data of the brother, but it was not possible; an X-ray of the time when the diagnosis was made, provided by the same relative, was recovered.
Once known this information, the other siblings were called for an appointment, but it only was possible to access the radiograph of the oldest sister, which did not show any abnormality.
Ethical responsibilities
Protection of people and animals. The authors declare that no experiments were performed on human beings or animals for this research.
Data confidentiality. The authors state that patient data do not appear in this article.
Right to privacy and informed consent. The authors state that patient data do not appear in this article.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
To Dr. Alejandro Ruíz, MD., specialist in Internal Medicine and Pulmonology of the Simon Bolivar Hospital; Dr. Edgar Parra, MD., specialist in Pathology of the National Institute of Health, and Dr. Fernando Páramo, MD., specialist in Internal Medicine of the Simon Bolivar Hospital, Service of Pathology of the Simon Bolivar Hospital.
References
1. Mariotta S, Ricci A, Papale M, de Clementi F, Sposato B, Guidi L, et al. Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:173-81. [ Links ]
2. Castellana G, Lamorgese V.Pulmonary alveolar microlithiasis. World cases and review of the literature. Respiration. 2003;70:549-55. [ Links ]
3. Prakash UB. Pulmonary alveolar microlithiasis. Semin Respir Crit Care Med. 2002;23:103-13. [ Links ]
4. Abdel-Hakim M, El-Mallah S, Hashem M, Abdel-Halim S. Pulmonary alveolar microlithiasis. Thorax. 1959;14:263. [ Links ]
5. Lauta VM. Pulmonary alveolar microlithiasis: an overview of clinical and pathological features together with possible therapies. Respir Med. 2003;97:1081-5. [ Links ]
6. Viswanathan R. Pulmonary alveolar microlithiasis. Thorax. 1962;17:251. [ Links ]
7. Al-Damluji SF, Al-Omari MM, Al-Fakhri S. Pulmonary alveolar microlithiasis in two siblings from Iraq. Br J Dis Chest. 1973;67:246-52. [ Links ]
8. Tachibana T, Hagiwara K, Johkoh T. Pulmonary alveolar microlithiasis: review and management. Curr Opin Pulm Med. 2009;15:486-90. [ Links ]
9. Ortakóylü G, Ketenci A, Ayse B, Emel C, Nur Ü. Pulmonary alveolar microlithiasis. Turkish Resp J. 2006;7:034-37. [ Links ]
10. Ucan E, Keyf A, Aydilek R, Yalcin Z, Sebit S, Kudu M, et al. Pulmonary alveolar microlithiasis: review of Turkish reports. Thorax. 1993;48:171. [ Links ]
11. Mariotta S, Guidi L, Papale M, Ricci A, Bisetti A. Pulmonary alveolar microlithiasis: review of Italian reports. Eur J Epidemiol. 1997;13:587-90. [ Links ]
12. Castellana G, Gentil M, Castellana R, Fiorente P, Lamorgese V. Pulmonary alveolar microlithiasis: clinical features, evolution of the phenotype, and review of the literature. Am J Med Genet. 2002;111:220-4. [ Links ]
13. Onadeko BO, Abioye AA, Beetlestone CA, Adetuyibi A, Cooke AR, Sofowora EO. Pulmonary alveolar microlithiasis. Postgrad Med J. 1977;53:1-7. [ Links ]
14. Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 2002;165:1654-69. [ Links ]
15. Yesner R. Pulmonary alveolar microlithiasis revisited. N Engl J Med USA. 2003:84-5 [ [ Links ]author reply 5].
16. Caffrey PR, Altman RS. Pulmonary alveolar microlitbiasis occurring in premature twins. J Pediatr. 1965;66:758-63. [ Links ]
17. Yang SP, Lin CC. Pulmonary alveolar microlithiasis. Dis Chest. 1963;44:163-7. [ Links ]
18. Esguerra Gómez G, Lichtem-Berger E, Santamaría A, Carvajal L, Jiménez-Penuela B, Saaibi E, et al. Familial pulmonary alveolar microlithiasis: four cases from Colombia. S.A.; is microlithiasis also environmental disease. Radiology. 1959;72:550-61. [ Links ]
19. Esguerra Gómez G. Pulmonary alveolar microlithiasis in Colombia. Rev Fac Med Univ Nac Colomb. 1961;29: 5-22. [ Links ]
20. Vallejo FJ, Vallejo A. Microlitiasis alveolar pulmonar. Acta Méd Colomb. 2007;32:223-6. [ Links ]
21. Gutiérrez-González C, Angarita J-I, Ballesteros JG, Triana LC. Reporte de caso: microlitiasis alveolar pulmonary. In: Póster, XIII Congreso colombiano de Neumología. Rev Colomb Neumol: Asociación Colombiana de Neumología y cirugia de Tórax. 2009. p. 31. [ Links ]
22. Sears MR, Chang AR, Taylor AJ. Pulmonary alveolar microlithiasis. Thorax. 1971;26:704-11. [ Links ]
23. Bhatia J, Thind G. Pulmonary alveolar microlithiasis. Indian J Tuberc. 1976;33:110. [ Links ]
24. Wallis C, Whitehead B, Malone M, Dinwiddie R. Pulmonary alveolar microlithiasis in childhood: diagnosis by transbronchial biopsy. Pediatr Pulmonol. 1996;21: 62-4. [ Links ]
25. Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004;447: 763-7. [ Links ]
26. Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650-6. [ Links ]
27. Huqun, Izumi S, Miyazawa H, Ishii K, Uchiyama B, Ishida T, et al. Mutations in the SLC34A2 gene are associated with pulmonary alveolar microlithiasis. Am J Respir Crit Care Med. 2007;175:263-8. [ Links ]
28. Virkki LV, Biber J, Murer H, Forster IC. Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol. 2007;293:F643-54. [ Links ]
29. Ishihara Y, Hagiwara K, Zen K, Huqun, Hosokawa Y, Natsuhara A. A case of pulmonary alveolar microlithiasis with an intragenetic deletion in SLC34A2 detected by a genome-wide SNP study. Thorax. 2009;64:365-7. [ Links ]
30. Akyildiz EU, Ursavas A, Ogur U. Pulmonary alveolar microlithiasis in a textile worker. Ann Thorac Surg. 2009;88:e22-4. [ Links ]
31. Giménez C, Pinet C, Fraticelli A, Barlesi F, Orehek J, Astoul P. Alveolar microlithiasis and immunological disorders. Int J Tuberc Lung Dis. 2004;8:803. [ Links ]
32. Chinachoti N, Tangchai P. Pulmonary alveolar microlithiasis associated with the inhalation of snuff in Thailand. Dis Chest. 1957;32:687-9. [ Links ]
33. Patro S, Kar C. Pulmonary alveolar microlithiasis. Ind J Radiol Imag. 2006;16:879. [ Links ]
34. Türktas I, Saribas S, Balkanci F. Pulmonary alveolar microlithiasis presenting with chronic cough. Postgrad Med J. 1993;69:70. [ Links ]
35. Deniz O, Ors F, Tozkoparan E, Ozcan A, Gumus S, Bozlar U, et al. High resolution computed tomographic features of pulmonary alveolar microlithiasis. Eur J Radiol. 2005;55:452-60. [ Links ]
36. Wang HY, Shen HH, Jiang ZN. Pulmonary alveolar microlithiasis: report of four familial cases. Chin Med J (Engl). 2004;117:950-2. [ Links ]
37. Thomson WB. Pulmonary alveolar microlithiasis. Thorax. 1959;14:76-81. [ Links ]
38. Qublan HS, Athamneh I, Al-Kaisi NS. Azoospermia associated with testicular and pulmonary microlithiasis. J Diag Med Sonogr. 2003;19:192. [ Links ]
39. Coetzee T. Pulmonary alveolar microlithiasis with involvement of the sympathetic nervous system and gonads. Thorax. 1970;25:637-42. [ Links ]
40. Giallauria F, Giallauria G. Pulmonary alveolar microlithiasis. Thorax. 2011;66:840. [ Links ]
41. Brandenburg VM, Schubert H. Pulmonary alveolar microlithiasis. N Engl J Med. 2003;348:1555-60. [ Links ]
42. Kanat F, Teke T, Imecik O. Pulmonary alveolar microlithiasis with epididymal and periurethral calcifications causing obstructive azospermia. Int J Tuber Lung Disease: Off J Int Union Tuber Lung Dis. 2004;8:1275. [ Links ]
43. Emri S, Coplu L, Selcuk ZT, Sahin AA, Baris YI. Hypertrophic pulmonary osteoarthropathy in a patient with pulmonary alveolar microlithiasis. Thorax. 1991;46:145-6. 2016;23(2):115-120. [ Links ]
44. Glynos C, Papathanasiou N, Nikoloutsou I, Stathopoulos GT. Pulmonary alveolar microlithiasis. Am J Respir Crit Care Med. 2011;184:740. [ Links ]
45. Miro JM, Moreno A, Coca A, Segura F, Soriano E. Pulmonary alveolar microlithiasis with an unusual radiological pattern. Br J Dis Chest. 1982;76:91-6. [ Links ]
46. Balikian JP, Fuleihan FJ, Nucho CN. Pulmonary alveolar microlithiasis. Report of five cases with special reference to roentgen manifestations. Am J Roentgenol Radium Ther Nucl Med. 1968;103:509-18. [ Links ]
47. Roca Vanaclocha Y, Narváez JA, Pozuelo C, Monés L. [Alveolar microlithiasis: an uncommon cause of the crazy paving pattern]. Radiología. 2008;50:75-8. [ Links ]
48. Xaubet A, Ancochea J, Blanquer R, Montero C, Morell F, Rodríguez Becerra E, et al. Diagnóstico y tratamiento de las enfermedades pulmonares intersticiales difusas. Arch Bronconeumol. 2003;39:580-600. [ Links ]
49. Gayathri B, Keerthivasan S. Pulmonary alveolar microlithiasis. J Medicine. 2011:55-7. [ Links ]
50. Razi E, Ehteram H, Talari H. Pulmonary alveolar microlithiasis. Iran J Radiol. 2006;3:3. [ Links ]
51. Gasparetto EL, Tazoniero P, Escuissato DL, Marchiori E, Frare ESRL, Sakamoto D. Pulmonary alveolar microlithiasis presenting with crazy-paving pattern on high resolution CT. Br J Radiol. 2004;77:974-6. [ Links ]
52. Cluzel P, Grenier P, Bernadac P, Laurent F, Picard JD. Pulmonary alveolar microlithiasis: CT findings. J Comput Assist Tomogr. 1991;15:938-42. [ Links ]
53. Batchelor TJ, Ngaage DL, McGivern DV, Cowen ME. Surgical management of pneumothorax in pulmonary alveolar microlithiasis. Ann Thorac Surg. 2007;84:290-2. [ Links ]
54. Marchiori E, Franquet T, Gasparett TD, Goncalves LP, Escuissato DL. Consolidation with diffuse or focal high attenuation: computed tomography findings. J Thorac Imaging. 2008;23:298-304. [ Links ]
55. Padhy AK, Sharma SK, Vijayaraghavan M, Gopinath PG. Role of bone scanning in pulmonary alveolar microlithiasis. Report of two cases. Clin Nucl Med. 1991;16:458-60. [ Links ]
56. Monabati A, Ghayumi MA, Kumar PV. Familial pulmonary alveolar microlithiasis diagnosed by bronchoalveolar lavage. A case report. Acta Cytol. 2007;51:80-2. [ Links ]
57. Barnard NJ, Crocker PR, Blainey AD, Davies RJ, Ell SR, Levison DA. Pulmonary alveolar microlithiasis. A new analytical approach. Histopathology. 1987;11:639-45. [ Links ]
58. Greenberg MJ. Miliary shadows in the lungs due to microlithiasis alveolaris pulmonum. Thorax. 1957;12:171-1. [ Links ]
59. Kang HW, Kim TO, Oh IJ, Kim YI, Lim SC, Choi YD, et al. A case of pulmonary alveolar microlithiasis. J Korean Med Sci. 2011;26:1391-3. [ Links ]
60. Palombini BC, da Silva Porto N, Wallau CU, Camargo JJ. Bronchopulmonary lavage in alveolar microlithiasis. Chest. 1981;80:242-3. [ Links ]
61. Barbolini G, Rossi G, Bisetti A. Pulmonary alveolar microlithiasis. N Engl J Med U S A. 2002:69-70. [ Links ]
62. Fiore CA, Duarte Sotelo L, Vázquez S, Mirofsky M, Bauni C. Pulmonary alveolar microlithiasis. Medicina (B Aires). 2006;66:50. [ Links ]
63. Gowrinath K, Warrier AR. Pulmonary alveolar microlithiasis. Lung India. 2006;23:42. [ Links ]
64. Shigemura N, Bermudez C, Hattler BG, Johnson B, Cresp M, Pilewski J, et al. Lung transplantation for pulmonary alveolar microlithiasis. J Thorac Cardiovasc Surg. 2010;139:e50-2. [ Links ]











 texto en
texto en