Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Reumatología
Print version ISSN 0121-8123
Rev.Colomb.Reumatol. vol.24 no.1 Bogotá Jan./Mar. 2017
https://doi.org/10.1016/j.rcreu.2016.06.002
Case report
Possible ocular adverse effects attributable to bisphosphonate therapy: Two case reports ☆
a Facultad de Medicina, Universidad de los Andes, Bogotá, Colombia
b Medicina Interna, Endocrinología, Hospital Universitario Fundación Santa Fe de Bogotá, Bogotá, Colombia
Bisphosphonates are used in the management of diseases characterized by an increase in bone resorption such as osteoporosis, metatasic bone disease, malignant hypercalcemia among others. It has been reported that the use of IV bisphosphonates as zoledronate and pamidronate generate ocular adverse effects by an acute phase reaction mediated by an increase of interleukin 6 (IL-6) and tumoral necrosis factor (TNF-a). We present 2 cases, a woman 71 years old and a 67 years old man that received therapy with bisphosphonates and 24 to 72hours later they presented an anterior uveitis.
Keywords: Uveitis; Bisphosphonates; Zoledronic acid; Adverse effect
Los bifosfonatos se utilizan para el manejo de enfermedades con incremento de la resorción ósea como la osteoporosis, la enfermedad metastásica ósea y la hipercalcemia maligna, entre otras patologías. En los últimos años se ha reportado que el uso de bifosfonatos intravenosos como el zoledronato y el pamidronato pueden generar efectos adversos oculares, ocasionados por una reacción de fase aguda mediada por la interleucina-6 (IL-6) y factor de necrosis tumoral alfa (TNF-a). Se reportan 2 casos (una mujer de 71 años y un hombre de 67 años) que entre las 24 a 72 h después de recibir terapia con zoledronato presentaron una uveítis anterior.
Palabras clave: Uveítis; Bifosfonatos; Ácido zoledrónico; Efecto adverso
Introduction
Bisphosphonates have been used in subjects with confirmed diagnosis of osteoporosis, Paget's disease,1 neoplastic diseases with bone metastases, malignant hypercalcemia and other pathologies such as osteogenesis imperfecta.2 In the world, the prescription of this family of drugs has increased; an example of this is that from 2002 to 2007 the use of thera pies with oral bisphosphonates reached 52.7 million formulas, and with intravenous bisphosphonates, in the United States, from 2002 to 2010 it increased by 561,000 doses.3 In Spain it has been also observed an increase in the prescription of bisphosphonates up to double between 1998 and 2003.4
It has been reported that the use of intravenous and oral bisphosphonates can generate ocular adverse effects by a transient increase in pyrogenic cytokines and an increased production of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) mediated by 7/8 T lymphocytes.5 The most com mon adverse effects are anterior uveitis, episcleritis, scleritis and orbital inflammatory disease.6
The objective of this publication is to report 2 cases of pos sible secondary effects of bisphosphonates at the ocular level, especially of zoledronic acid.
Case presentation
Case #1
A 74-year-old woman with presence of high bone alkaline phosphatase despite the correction of the 25 OH-vitamin D deficiency, normal calcemia, normal phosphatemia, normal 1,25-dihydroxyvitamin D, normal PTH, dietary and supple mental calcium intake; ruling out clinically and radiologically an osteomalacia and considering another metabolic bone dis ease. With DXA densitometry compatible with osteoporosis according to the criteria of the World Health Organization (WHO), it was prescribed zoledronic acid, 5 mg intravenously, to be applied during 15 min. 48 h after having received the infu sion, the patient presented pain in the right eye accompanied by conjunctival hyperemia with dilated episcleral vessels. At the ophthalmological examination the patient presented pos itive Tyndall and Flare (++), nuclear sclerosis and a decrease in visual acuity in the right eye 20/200. In the fundus of the eye were observed excavated oval papillae, a macula with slight decrease in brightness without the presence of infection foci, and an ocular pressure of 12 mmHg; it was diagnosed an acute anterior uveitis (AAU) of the right eye and it was prescribed prednisolone acetate, tropicamide and drops of hydroxypropyl-methylcellulose. The symptomatology improved after treatment within the following 8 days and did not leave any sequels.
As relevant antecedents, the patient has controlled high blood pressure, hyperthyroidism due to toxic multinodular goiter managed with iodine 131 mCI with successful response, type 1 gastric neuroendocrine tumor with histological grade 2 according to the WHO classification, recurrent, associated with autoimmunity, managed with subtotal gastrectomy; monostotic dysplasia with involvement of the frontal bone that required management with osteosynthesis; café-au-lait spots; and in this context the McCune-Albright syndrome is suspected, with a negative mutation for the GNAS gene, to which is pending to carry out the whole gene sequenc ing. The patient has presented drug allergy to dapsone. As concomitant medication the patient was receiving levothyroxine, cholecalciferol and calcium carbonate. After the adverse event, the patient was taken to the medical board, where it was decided to control the decrease in bone mass with denosumab 60 mg subcutaneously, every 6 months, but the patient did not accept; at the present time she continues under close surveillance with bone alkaline phosphatase, densitometries and clinical examinations.
Case #2
A 67-year-old man who enters the emergency department with a clinical picture of 72 h of evolution consisting in bilat eral eye pain, red eye, photophobia without alteration of visual acuity following the application of zoledronic acid (4mg) for the management of polyostotic disease associated with prostate carcinoma. The ophthalmological exam reported generalized conjunctival hyperemia, intraocular pressure of 14 mmHg, positive Tyndall and Flare (++/+), round papillae with well-defined edges and a healthy macula. In addition, he reported hyperthermia of 38 degrees during 2 days, dys pnea, myalgias and arthralgias with limitation of movement, unsteady gait and prostration that lasted for a month. It was diagnosed a bilateral AAU and topical cyclopentolate and prednisolone F were prescribed. The patient recovered within a month without sequelae.
As relevant antecedents the patient has a stage IV prostate carcinoma diagnosed in October 2014, with metastatic bone involvement (spine and hip) managed with hormonal block ade, bilateral phakectomy with intraocular lenses, retinal detachment in left eye, primary hypothyroidism, low bone mass and dyslipidemia. As concomitant medication the patient was receiving cholecalciferol and goserelin acetate. The patient has no history of allergies to any medication. After the adverse event the prescription was changed for denosumab, in part because the patient refused to use the drug again.
Discussion
Bisphosphonates decrease the rhythm of bone resorption and delay the bone loss7 by 2 mechanisms: (1) Non-nitrogen containing bisphosphonates have similarity to pyrophos phate, being incorporated into the molecule of adenosine triphosphate (ATP), producing non-hydrolizable ATP, which accumulates and inhibits multiple ATP-dependent cellular processes and, finally, causes toxicity. (2) Nitrogen containing bisphosphonates inhibit the enzyme farnesyl pyrophosphate (FPP) synthase, involved in the mevalonate pathway, which is necessary for the construction of the cytoskeleton and the survival of the osteoclast.8
Among the options of management with bisphosphonates, intravenous bisphosphonates have emerged as an interest ing choice due to their posology and potency.9-11 Zoledronic acid is one of the most commonly used intravenous bisphos phonates for the management of osteoporosis, accompanied by pamidronate, alendronate and risedronate.3,9 The mechanism of action of this family of bisphosphonates with a nitrogen group is the inhibition of the enzyme FPP synthase, which is involved in the mevalonate pathway. This pathway is critical for the production of sterols, such as cholesterol and isoprenoid lipids. These sterols are essential for protein post-translational modifications. Examples of this are the modifications to proteins bound to guanosine-triphosphate such as Rab, Rac and Rho. These proteins are involved in central processes of regulation of cellular activities of the osteoclast such as fiber assembly, membrane folding and sur vival. Their alteration generates, finally, the apoptosis of the osteoclast and prevents bone resorption.8
It has been observed that in certain patients the use of bisphosphonates generates an acute phase reaction (APR) mediated by the increase in the production of IL-6 and TNF-α mediated by γ/δ T lymphocytes.5,12 This reaction generates systemic symptoms such as fever, myalgias and arthralgias, resembling a flu episode.6,11 The activation of these lymphocytes is mediated by monocytes that in the pres ence of the drug accumulate isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), activating the γ/δ lymphocytes directly by contact.13
Through the APR, bisphosphonates can cause serious ocular adverse effects.14,15 The most commonly associated bisphosphonates are the intravenous, such as pamidronate and zoledronate16; however, it is important to highlight that some non-nitrogen-containing bisphosphonates, such as clodronate, can also generate ocular adverse effects.17 The most commonly reported ocular pathologies are scleritis, conjunc tivitis and uveitis14; and less frequent blepharitis, synechiae, subconjunctival hemorrhage,33 intraocular hypertension, and ischemic optic neuropathy.34 Orbital inflammation and myositis can be seen in ocular imaging with ultrasound, magnetic resonance or computed tomography.34 These adverse effects may affect the eyes bilaterally or unilaterally, usually with a mild to moderate severity; there is reversibility without sequelae within the first month if a timely management is implemented. With the use of intravenous bisphosphonates the toxicity occurs usually within 48-72 h after the application of the drug, but in certain cases the adverse effect may appear after 2 weeks. With the use of oral bisphosphonate, ocular tox icity may appear between 2 and 3 months of use.15 It is still not clear why this APR affects primarily the eyes. An in vitro study of human retinal cells (hRPE) demonstrated that, in the pres ence of alendronate and etidronate, these cells increase the production of inflammatory cytokines such as IL-6 and IL-8 and decrease angiogenic factors such as eotaxin and the basic fibroblast growth factor (bFGF).18 However, the biological plau sibility is still unclear and further studies in this regard should be carried out. In addition, it has been observed that patients under therapy with bisphosphonates may have leukopenia and elevated C-reactive protein (CRP), factors that can promote the development of these adverse effects (Fig. 1).19
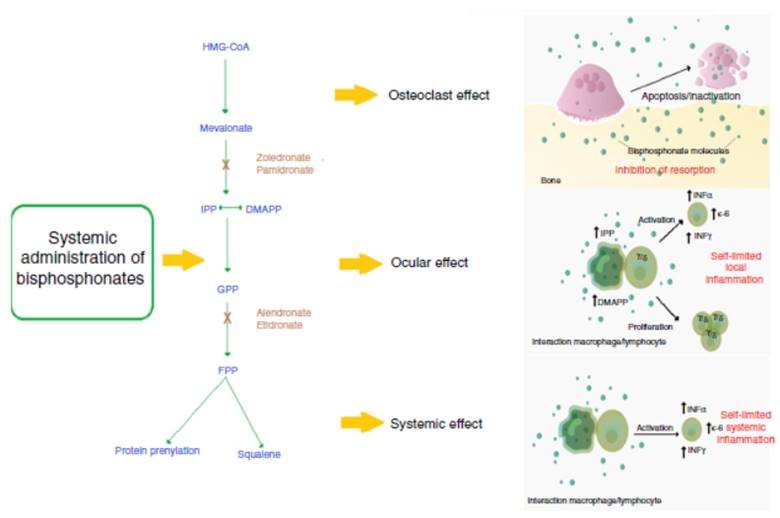
Fig. 1 Mechanism of action of nitrogen-containing bisphosphonates and their relationship with the ocular adverse event. The systemic nitrogen-containing bisphosphonates lead to alteration in the mevalonate pathway generating the apoptosis of the osteoclast; this causes inhibition of bone resorption which is the expected therapeutic effect. Presumed mechanism of the acute phase reaction: the use of bisphosphonates generates a self-limited systemic inflammation mediated by increased serum levels of IL-6, IFN-γ and TNF-α. It is described that the cell populations probably implied in the mechanism of action are monocytes/macrophages and T lymphocytes. Presumed mechanism of the ocular adverse effect: the use of nitrogen-containing bisphosphonates generates the sensitization of circulating monocytes by accumulation of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which by a mechanism of direct contact activates a subpopulation of T lymphocytes (γ/δ) that produces a self-limited local inflammation in the eye mediated by IL-6, TNF-α and IFN-γ.
DMAPP: dimethylallyl pyrophosphate; FPP: farnesil pyrophosphate; GPP: geranyl pyrophosphate; HMG-CoA: 3-hydroxy-3 methylguanylyl coenzyme A; IFN-γ: interferon gamma; IL-6: interleukin 6; IPP: isopentenyl pyrophosphate; TNF-α: tumor necrosis factor alpha.
Among the ocular signs and symptoms found in multiple reports the most common are conjunctival chemosis, conjunc tival hyperemia, edema of the lens, deficit in ocular motility, anterior uveitis, anterior scleritis, proptosis, blurred vision, diplopia and pain.16,20,21
In a retrospective cohort study it was observed that nitrogen-containing oral and intravenous bisphosphonates can cause ocular adverse effects, and it is estimated that the incidence of AAU in the users is 29 cases/100,000 people, with a relative risk (RR) of having the condition due to their use of 1.45 (CI 95%: 1.25-1.68).22
A prospective, double-masked placebo-controlled clinical study showed that the incidence of AAU was 0.8% applying 5 mg of zoledronic acid in postmenopausal patients (n = 2001) with low bone mass. However, the authors report that the true incidence may be much higher, since the data obtained are only of patients who consulted the ophthalmologist.15 A new prospective double-masked, placebo-controlled study for prevention of vertebral fracture with 1054 postmenopausal women and under active surveillance of the ocular adverse effects found that the incidence of AAU was 1.1% with 5mg of zoledronic acid, 1/13 women had positive p-ANCA, 1/13 women had positive ANA (but negative ENA) and 3/13 women had positive HLA-27.33
Patients with osteoporosis or polyostotic diseases usually receive polypharmacy, and it is important to emphasize that bisphosphonates are not the only medicines that cause ocu lar adverse events. Other drugs with recognized association are cidofivir, anti-vascular endothelium growth factor (VEGF) agents, rifabutin, sulphonamides, metipranolol, brimonidine, among others (Table 1).16 For this reason exist the assessment by the WHO23,24 and the criteria of Naranjo et al.25 that quan tify the probability that an adverse effect is caused by the drug and not by something different. In studies of drug-induced uveitis is observed that bisphosphonates are among the few drugs that have a score of 10 in the Naranjo scale and met all the requirements for causality of the WHO, demonstrat ing their definitive association with the adverse effect.16,19 In the 2 cases exposed, the criterion of multiple exposure to the drug could not be met since the dose of medication was not repeated; however, the pattern described and the absence of other explanations suggest that the uveitis was related with the use of zoledronic acid, without disregarding that the patients have other conditions that favor the risk for this adverse effect.
Table 1 - Drugs with confirmed causation of anterior uveitis both by the criteria of Naranjo and by causality evaluation of the WHO.
| Drugs associated with anterior uveitis | |||
| Drug | Criteria of Naranjo | Evaluation of causality of the WHO | |
| Score | Causality | Probable | |
| Cidofovir | 11 | Definitive | Possible |
| Anti-VEGF | 11 | Definitive | Probable |
| Rifabutin | 10 | Definitive | Probable |
| Sulphonamides | 10 | Definitive | Definitive |
| Bisphosphonates | 10 | Definitive | Definitive |
| Metipranolol | 10 | Definitive | Probable |
| Glucocorticosteroids | 9 | Definitive | Possible |
| Brimonidine | 9 | Definitive | Possible |
| Prostaglandin analogues | 9 | Definitive | Possible |
| BCG vaccine | 9 | Probable | Possible |
| TNF-a antagonists | 7 | Probable | Probable (etanercept) |
| Triamcinolone acetonide | 7 | Probable | Possible (adalimumab, infliximab) |
| Influenza vaccine | 7 | Probable | Possible |
| Triple viral vaccine | 7 | Probable | Possible |
| Hepatitis B vaccine | 6 | Probable | Possible |
| Fluoroquinolones | 6 | Probable | Possible |
| Diethylcarbamazine | 5 | Probable | Possible |
| Chickenpox vaccine | 4 | Possible | Possible |
| Taken and modified from Cordero-Coma et al.16 | |||
In the case reports described in the literature, a sig nificant number of subjects who exhibit ocular adverse events associated with bisphosphonates have comorbidities of autoimmune basis such as Type 1 diabetes mellitus, ankylosing spondylitis, inflammatory bowel disease or sarcoidosis.22,26,27 The following have been described as risk factors for APR: age of 60-70 years, active back pain, cigarette smoking, use of calcitonin, being a non-Japanese Asian or Pacific Islander and previous use of bisphosphonates.27 How ever, the possible association between autoimmunity and uveitis has not been described. It is important, then, to con duct population studies to define whether an autoimmune disease, polymorphic variants of the HLA major histocompatibility complex or the cytokines involved in the inflammatory cascade constitute risk factors for ocular adverse effects. In our 2 cases, the subjects were between 60 and 70 years old and had hypothyroidism, which is usually an autoimmune disease.
There is no solid evidence related to the management of an ocular adverse event because the reports are very scarce; how ever, we recommend to educate the patient about the possible ocular side effects and to discuss the change of mechanism of action of the drug for osteoporosis, due to the possible recurrence of the ophthalmological secondary effects. Nev ertheless, not all patients who are re-exposed to the drug have a recurrence of the ophthalmological alteration.28 In case of discontinuing the use of bisphosphonate, the alternative is to change the therapeutic group if the patient has a high risk of fracture. Currently, there are several options available, such as parathyroid hormone analogs (teriparatide), which are useful in the patients with fractures due to the use of corticosteroids; denosumab (anti-RANK-L ligand antibody), which is an option for the patient with cancer; strontium ranelate, estrogen receptor modulators and cathepsin K inhibitors.29 The patients with low risk of fractures can be managed with cholecalciferol and dietary or supplemental calcium accom panied by exercise and a fall prevention program.
For the management of the ocular adverse effect it is recommended to discontinue the treatment, to check if the patient has concomitant medications associated with ocular toxicity, to evaluate risk factors (among them autoimmunity) and to carry out the ophthalmological evaluation and symp tomatic management with topical steroids and cycloplegics, for a few weeks, to control the inflammation and improve vision (Fig. 2). In some severe cases arises the need to use systemic steroids or immunosuppressive agents based on the severity of the inflammatory process.16
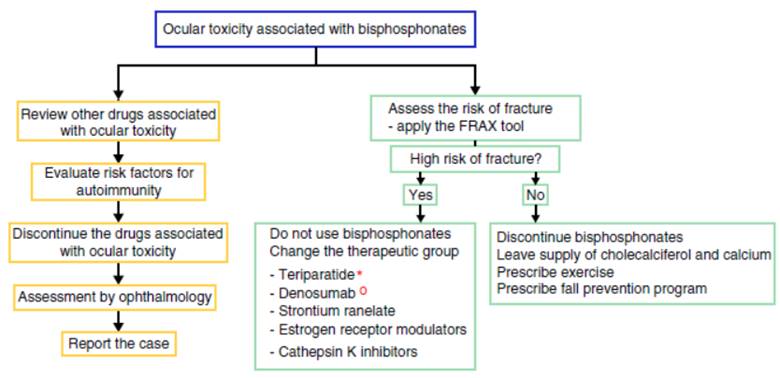
Figure 2 Proposed algorithm for the evaluation of the ocular adverse effect and for the therapeutic management of patients with high or low risk of fracture who present ocular adverse effects with bisphosphonates. In the left flowchart it is recommended to establish causality and in the right flowchart it is recommended to evaluate the risk of fracture and to define if it requires active therapy for osteoporosis or the disease that deserves the use of bisphosphonates. * The use of teriparatide is posed in the context of glucocorticoid-induced osteoporosis. ° The use of denosumab is posed in the context of polyostotic disease in solid tumor.
Conclusion
The safety and efficacy of the therapy with bisphosphonates are well documented.11,30-32 However, the increased use of intravenous bisphosphonates and their possible association with reversible ocular adverse effects requires population studies in this regard and the prospective evaluation of the reported cases in order to define the best management and the long term sequelae.
Ethical disclosures
Protection of human and animals subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or sub jects mentioned in the article. The corresponding author is in possession of this document.
REFERENCES
1. Hosking D, Lyles K, Brown JP, Fraser WD, Miller P, Curiel MD, et al. Long-term control of bone turnover in Paget's disease with zoledronic acid and risedronate. J Bone Miner. 2007;22:142-8. [ Links ]
2. George S, Weber DR, Kaplan P, Hummel K, Monk HM, Levine MA. Short-term safety of zoledronic acid in young patients with bone disorders: An extensive institutional experience. J Clin Endocrinol Metab. 2015;100:4163-71. [ Links ]
3. Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002-2012. Bone. 2013;57:423-8. [ Links ]
4. García del Pozo J, García del Pozo V, Sáinz Gil M, Carvajal García-Pando A. Use of medication for osteoporosis in Spain. Aten Primaria. 2004;33:104. [ Links ]
5. Diel IJ, Bergner R, Grótz KA. Adverse effects of bisphosphonates: Current issues. J Support Oncol.2007;5:475-82. [ Links ]
6. Papapetrou PD. Bisphosphonate-associated adverse events. Horm Athens Greece. 2009;8:96-110. [ Links ]
7. Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med.2010;363:2027-35. [ Links ]
8. Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-45. [ Links ]
9. Curtis JR, Yun H, Matthews R, Saag KG, Delzell E. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res. 2012;64:1054-60. [ Links ]
10. Russell RGG. Determinants of structure-function relationships among bisphosphonates. Bone. 2007;40:S21-5. [ Links ]
11. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-22. [ Links ]
12. Dicuonzo G, Vincenzi B, Santini D, Avvisati G, Rocci L, Battistoni F, et al. Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine. 2003;23:649-54. [ Links ]
13. Roelofs AJ, Jauhiainen M, Mónkkónen H, Rogers MJ, Mónkkónen J, Thompson K. Peripheral blood monocytes are responsible for gamma-delta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245-50. [ Links ]
14. Fraunfelder FW, Fraunfelder FT, Jensvold B. Scleritis and other ocular side effects associated with pamidronate disodium. Am J Ophthalmol. 2003;135:219-22. [ Links ]
15. Patel DV, Horne A, House M, Reid IR, McGhee CNJ. The incidence of acute anterior uveitis after intravenous zoledronate. Ophthalmology. 2013;120:773-6. [ Links ]
16. Cordero-Coma M, Salazar-Méndez R, Garzo-García I, Yilmaz T. Drug-induced uveitis. Expert Opin Drug Saf. 2015;14: 111-26. [ Links ]
17. Fietta P, Manganelli P, Lodigiani L. Clodronate induced uveitis. Ann Rheum Dis. 2003;62:378. [ Links ]
18. Or C, Cui J, Matsubara J, Forooghian F. Pro-inflammatory and anti-angiogenic effects of bisphosphonates on human cultured retinal pigment epithelial cells. Br J Ophthalmol. 2013;97:1074-8. [ Links ]
19. Moorthy RS, London NJS, Garg SJ, Cunningham ET. Drug-induced uveitis. Curr Opin Ophthalmol. 2013;24:589-97. [ Links ]
20. Peterson JD, Bedrossian EH. Bisphosphonate-associated orbital inflammation-a case report and review. Orbit Amst Neth. 2012;31:119-23. [ Links ]
21. McKague M, Jorgenson D, Buxton KA. Ocular side effects of bisphosphonates: A case report and literature review. Can Fam Physician. 2010;56:1015-7. [ Links ]
22. Etminan M, Forooghian F, Maberley D. Inflammatory ocular adverse events with the use of oral bisphosphonates: A retrospective cohort study. CMAJ Can Med Assoc J. 2012;184:E431-4. [ Links ]
23. Letter MIO/372/2(A). Geneva, Switzerland: World Health Organization; 1991. [ Links ]
24. Holloway K, Green T, Policy WHOD of ED and M. Drug and therapeutics committees: A practical guide. Les comités pharmaceutiques et thérapeutiques: guide pratique. Internet. 2003 [consultado 24 May 2015]. Disponible en: http://apps.who.int//iris/handle/10665/68553 [ Links ]
25. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-45. [ Links ]
26. Pazianas M, Clark EM, Eiken PA, Brixen K, Abrahamsen B. Inflammatory eye reactions in patients treated with bisphosphonates and other osteoporosis medications: Cohort analysis using a national prescription database. J Bone Miner. 2013;28:455-63. [ Links ]
27. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95:4380-7. [ Links ]
28. Patel DV, Horne A, Mihov B, Stewart A, Reid IR, McGhee CNJ. The effects of re-challenge in patients with a history of acute anterior uveitis following intravenous zoledronate. Calcif Tissue Int. 2015;97:58-61. [ Links ]
29. Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. Internet. [consultado 12 May 2015]. Disponible en: http://www.nature.com/nrendo/journal/vaop/ncurrent/full/nrendo.2015.71.html?WT.mc_id=FBK_NatureReviews [ Links ]
30. Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer. 2003;9:2394-9. [ Links ]
31. McKeage K, Plosker GL. Zoledronic acid: A pharmacoeconomic review of its use in the management of bone metastases. PharmacoEconomics. 2008;26:251-68. [ Links ]
32. Li EC, Davis LE. Zoledronic acid: A new parenteral bisphosphonate. Clin Ther. 2003;25:2669-708. [ Links ]
33. Patel DV, Bolland M, Nisa Z, Al-Abuwsi F, Singh M, Horne A, et al. Incidence of ocular side effects with intravenous zoledronate: secondary analysis of a randomized controlled trial. Osteoporos Int. 2015;26:499-503. [ Links ]
34. Pirbhai A, Rajak SN, Goold LA, Cunneen TS, Wilcsek G, Martin P, et al. Bisphosphonate-Induced Orbital Inflammation: A Case Series and Review. Orbit. 2015;34:331-5. [ Links ]
Received: June 16, 2015; Accepted: June 06, 2016











 text in
text in 


