Introduction
Leflunomide is a drug that was initially studied as a treatment to prevent transplant rejection, however, its main current therapeutic use is for the treatment of rheumatoid arthritis (RA) as a disease modifying antirheumatic drug, although it is also used for the treatment of other rheumatic diseases such as psoriatic arthritis and systemic lupus erythematosus (SLE). Stevens Johnson syndrome (SJS) is a severe variant of erythema multiforme, in which there is a hypersensitivity reaction process that affects the skin and mucous membranes. We present the case of a patient with SLE, who had been receiving treatment with leflunomide, prescribed on an outpatient basis by her treating rheumatologist in her health care promoting entity. The patient attends our institution with a picture of SJS subsequent to the starting of the administration of leflunomide.
Case report
A 37-year-old woman who consulted to the emergency service of our institution due to a clinical picture of 4 days of evolution, characterized by conjunctival erythema and secretion, associated with generalized pruritus, malaise, painful lesions in oral mucosa, facial erythema and target lesions predominantly in lower limbs. As antecedents, the patient had been diagnosed with SLE 5 years ago, for which she had previously received management with prednisolone 5 mg/day and chloroquine 150 mg/day, with little improvement in her symptoms, and for this reason, 20 days before the consultation in the emergency service her treating physician started the administration of leflunomide, 20 mg/day. In addition to the foregoing, the patient has a history of acromegaly secondary to a pituitary adenoma resected 8 year ago, and occasionally takes omeprazole and calcium. Her toxic-allergic, family and gynecological antecedents are negative.
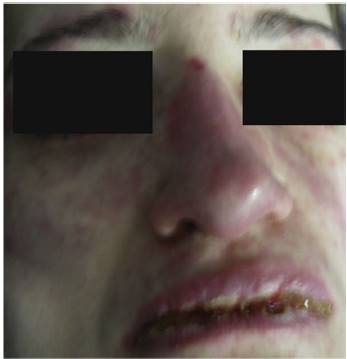
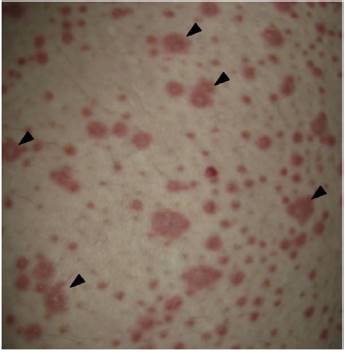
On physical examination the patient was in a fair general condition, afebrile, tachycardic (HR 100 x', BP 110/70 mm/Hg, RR 16 x') with marked bilateral conjunctival erythema associated with purulent discharge in conjunctival fornices and swollen eyelids. In the skin of the chicks, nasal dorsum and sidewalls, the patient presented erythematoedematous plaques; in the lips erosions covered by yellowish hematic crusts, and in the skin of the thorax and extremities, including palms and soles, countless erythematous and edematous papules, some of them bullseye-shaped (Figs. 1 and 2).
The patient was assessed by the dermatology service, and they considered a possible picture of SJS. A skin biopsy was performed, which showed findings characteristic of erythema multiforme, extensive damage of the interface of vacuolar type, associated with numerous diskeratocytes and subepidermal vesiculation. In the dermis, edema associated with perivascular inflammatory infiltrate of lymphocytes with some eosinophils (Figs. 3 and 4).
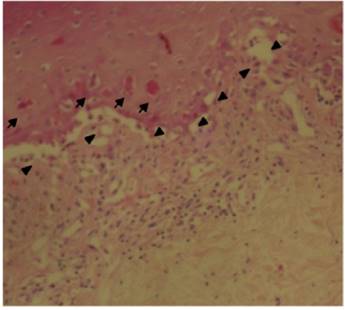
Fig. 3 Skin biopsy: epidermis with extensive damage of vacuolar interface (triangles), associated with dyskeratocytes (small arrows) and subepidermal vesiculation.
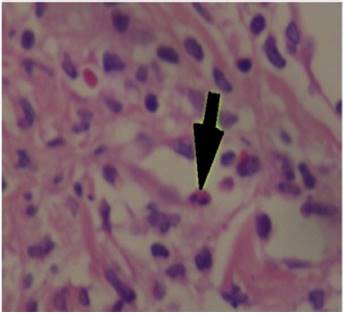
Fig. 4 Papillary dermis of edematous appearance with lymphocytary perivascular infiltrate with some eosinophils (large arrow).
Forty-eight hours after her admission the patient presented clear clinical deterioration, with fever, hypotension and tachycardia; the cutaneous lesions increased, with involvement of a greater body area, and on vaginal mucosa appeared tense blisters and lesions (Figs. 5 and 6). With these findings, the so extensive cutaneous and mucosal involvement and with a pathology or erythema multiforme that showed eosinophilic infiltrate, a diagnosis of SJS was made, and reviewing the history of the patient we considered that, since leflunomide was the only drug of recent start that she was being receiving, the dermatological picture was secondary to this antirheumatic agent. From the point of view of her autoimmune underlying disease, no lupus activity was documented, with normal complement, negative anti-DNA, blood count, nitrogen compounds and urinalysis within normal limits, and no clinical stigmata of SLE activity.
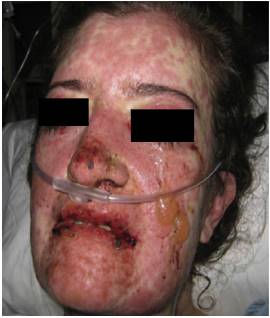
Fig. 5 Face skin with extensive involvement, with erythematoedematous plaques, tense blisters in left cheek and large erosions in eyelids, nasal dorsum and lips.
With the suspension of leflunomide and the supportive measures given during hospitalization for the management of the SJS, 5 days later, she presents a favorable evolution, with clear improvement of the cutaneous and mucosal lesions, as well as in the general involvement. The patient was discharged from our institution with antimalarial management and lowdose glucocorticoids.
Discussion
Leflunomide is an immunomodulatory drug widely used in ra1 demonstrating effectiveness similar to methotrexate2 and sulfasalazine,3 and for this reason is part of the disease modifying antirheumatic drugs (DMARDs) used for the treatment of RA. Subsequently, it was tested in animal models4 and in patients with SLE.5,6 With its use there have been described some cases of adverse reactions that compromise the hematologic, hepatic, respiratory and dermatologic systems.7
Leflunomide is a prodrug that is quickly absorbed and converted into its active form: A771726. The proposed immunoregulatory mechanism includes 3 points: A771726 inhibits an enzyme key in the synthesis of pyrimidines, the DHODH, and consequently the DNA and RNA synthesis, and thus, the cell proliferation,8 it inhibits the enzyme tyrosine kinase, an enzyme key in the translation of signals during the cell formation and division, inhibiting the production of proinflammatory cytokines such as tumor necrosis factor alpha and IL-17,9 and blocks TNF-mediated NF-Kb, thereby inhibiting the activation of T cells.10 The most common adverse effects of leflunomide include diarrhea, which occurs in approximately 20% of patients, in addition to nausea, dyspepsia, rash, weight loss, abnormalities in liver function tests,11 thrombocytopenia,12 interstitial pneumonia,13 peripheral neuropathy14 and cases of hypertriglyceridemia have also been documented.15 There have been reported some serious cutaneous reactions associated with leflunomide, among which are included toxic epidermal necrolysis,16,17 vasculitis18 and lichenoid reactions.19,20
SJS is a hypersensitivity reaction mediated by immune complexes, mainly associated with drugs, which typically affects the skin and mucosa being characterized by necrosis and detachment of the epidermis. The picture often begins with a maculopapular rash with target lesions, vesicles and blisters on skin and mucosa, with positive Nicolsky's sign and can evolve into toxic epidermal necrosis. It is associated with fever, headache, odynophagia, conjunctivitis and may present systemic complications such as acute kidney injury, hematological involvement and sepsis. The treatment is the immediate suspension of the drug which is causing the picture and hydric reanimation. Mortality is determined by the extent of the lesions. Bacteremia and sepsis increase mortality.21
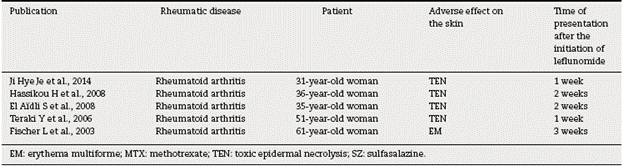
The cases of SJS and TEN reported in the literature are summarized in Table 1.
The aforementioned cases describe severe cases that are life-threatening and require to be managed in the intensive care unit. In all of them is carried out a thorough search of other possible causes such as bacterial or viral infections, which are always discarded and finally, given the pharmacological history of recent start of leflunomide, the picture is attributed to an adverse effect of the drug.
In Japan, in post-marketing records of 5163 patients treated with leflunomide are described 13 cases of SJS, however, cases of TEN are not recorded.17
Most cases appear within the 2 weeks following the start of leflunomide and it is recommended, given the long half-life of the drug (15-18 days), to administer cholestyramine in severe cutaneous reactions in order to avoid enterohepatic circulation and biliary recycling of the drug.
In the case of our patient, the picture of SJS was considered secondary to leflunomide, since this was the drug most recently started (3 weeks before the onset of the clinical picture); the antimalarial and the glucocorticoid were being prescribed from 5 years before, which considerably reduces the likelihood that the SJS would have been secondary to these 2 last drugs. In addition, the patient's clinical picture evolves toward resolution with the suspension of leflunomide and the patient is discharged receiving treatment with hydroxychloro-quine and prednisolone, without presenting new relapses of her hypersensitivity reaction.
Conclusion
A case of leflunomide-induced SJS in a patient with SLE is described. The clinical picture is an idiosyncratic lesion, impossible to predict, and therefore the treating physician must be attentive in the presence of signs of dermal toxicity in patients treated with leflunomide, especially in the first weeks after established the therapy. Despite the foregoing, leflunomide is a drug considered safe for the treatment of the rheumatic diseases for which it is indicated.
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.