Introduction
Systemic sclerosis (SSc) is a systemic autoimmune disease characterized by vasculopathy, inflammation, collagen deposits and fibrosis in the skin and internal organs such as the heart, lungs, gastrointestinal tract and kidneys.1-3 Renal complications are common in SSc.4 Chronic kidney disease (CKD) may occur; but also, high blood pressure, abnormal renal function or proteinuria are found in up to 60% of patients.5 In addition, between 5 and 15% of patients present with scleroderma renal crisis (SRC) which is the most serious complication of SSc.1 Before the advent of angiotensin-converting enzyme inhibitors (ACEI), short-term survival of SRC was less than 10%.6 SRC is defined as the new occurrence of accelerated arterial hypertension or rapidly progressive oliguric renal failure during SSc.7
The objective of this review is to describe the renal complications of scleroderma. A review of the literature was performed in the following databases: Medline (PubMed platform), Library Science Database (ProQuest platform), EMBASE (ProQuest platform), with the search terms: scleroderma, systemic sclerosis, systemic scleroderma, scleroderma renal, limited scleroderma, diffuse scleroderma, limited systemic scleroderma; in English language. Searches were conducted until April 2019.
Scleroderma renal crisis
SRC usually presents with severe arterial hypertension often described as accelerated or malignant, and acute renal failure (ARF). It is one of the most serious complications of SSc, occurring in approximately 5-15% of cases, of which 2% occur with the localized variety (SSlc) and 12% in the diffuse (SSdc).8 On the other hand, non-malignant arterial hypertension without uremia and abnormalities in the urine or mild uremia attributable to other factors in the absence of SRC are common in SSc and should not be confused with SRC.9
Pathophysiology
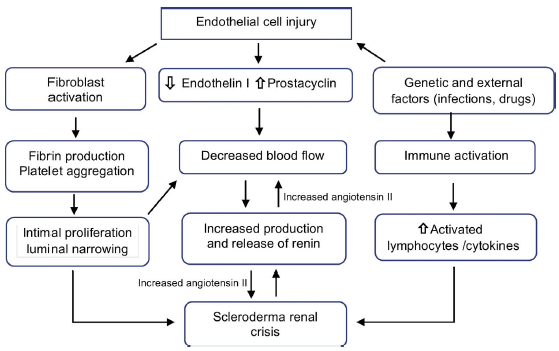
The primary process is the lesion of endothelial cells, which produces intimal thickening and proliferation of arched and interlobular arteries. Abnormal thickening of the vessel walls allows aggregation and adhesion of platelets. The release of platelet factors increases vascular permeability and may participate in the production of increased collagen and fibrin deposition contributing to the luminal narrowing (Fig. 1). These narrowed arterial vessels generate a decrease in renal perfusion, particularly in cortical blood flow, which added to the episodic vasospasm called "The renal Raynaud's phenomenon" demonstrated in the first classic studies of Cannon et al.,10 decreases the perfusion of the juxtaglomerular apparatus, generating its hyperplasia,11 promoting a marked plasma renin release, with activation of the renin-angiotensin-aldosterone system and production of angiotensin II which is a potent direct vasoconstrictor of vascular smooth muscle inducing malignant hypertension and rapidly progressive renal failure.12
Recent evidence showed that different fragments derived from the extracellular matrix play a role in the process of vascular remodeling since it is strictly related to apoptosis of endothelial cells.13 The increase in the levels of endothelin-1,14 soluble vascular adhesion molecules and soluble E-selectin15 has also been shown to be associated with renal crisis.
Although it was suspected that hormonal factors such as catecholamines, kinins and prostaglandyns are involved in the pathogenesis, only a few data support this hypothesis, suggesting that the pathophysiology of renal crisis is mainly due to the dysfunction of the factors that control the renal circulation,16 so that the SRC results from situations in which renal blood flow is compromised as in cardiac dysfunction,17,18 pregnancy, and alterations in blood volume and flow.19
Medications such as corticosteroids have been implicated for years in the development of SRC.20 A systematic review21 found a strong association between high doses of prednisone and renal crisis, but the exact pathophysiological relationship is unknown, although it is known that there is impaired endothelial function and inhibition of prostacyclin production, increasing angiotensin converting enzyme activity.
The patients most likely to receive steroids are those with early inflammatory disease having a higher risk of SRC. The severity of the disease and concomitant interventions can be confusing. But it is recommended that caution should be exercised when starting corticosteroids especially at high doses and in patients with early diffuse scleroderma. Other drugs, which cause vasospasm, and that have been associated with renal crises, are cyclosporine22 and tacrolimus.23
Predictors of scleroderma renal crisis
Predictive factors of future SRC include diffuse skin involvement, early disease with symptoms less than 4 years, rapid progression of skin thickening, presence of anti-RNA polymerase III antibody, new cardiac events (pericardial effusion, congestive heart failure or arrhythmias), recent onset anemia and a history of high doses of corticosteroids (greater than 15 mg of prednisone daily),7 as the main modifiable factor.10,24 The odds ratios for the development of SRC associated with exposure to corticosteroids in the last 1 or 3 months have been estimated at 24 (95% CI 3.0-193.8) and 17 (95% CI 2.1-144.0) respectively.25 On the contrary, the history of arterial hypertension is not usually associated with the development of SCR.26
Clinical manifestations
SRC is characterized by the acute onset of severe arterial hypertension often described as accelerated or malignant with ARF.13 Patients have characteristics that are seen in accelerated hypertension of any cause: headache, hypertensive retinopathy with blurred vision, or other encephalopathic symptoms, including seizures. Other clinical manifestations are microangiopathic hemolytic anemia (approximately 50% of cases), heart failure, pulmonary edema (due to increased afterload) and oliguria which can lead to salt and water retention. The presence of pericarditis, myocarditis, and arrhythmias conditions a worse prognosis.27 About 10% of these patients may present with normal blood pressure. However, blood pressure is probably high compared to their baseline blood pressure.28 In addition, it has been estimated that in 20% of patients, SRC precedes the diagnosis of SSc.29
Common laboratory abnormalities include increased serum creatinine (although in individuals with low baseline creatinine, serum levels may remain in a normal reference range), microangiopathic hemolytic anemia, thrombocytopenia (usually not less than 50,000/mm3), and high renin levels (30-40 times higher than normal). Urinalysis may present microscopic hematuria and granular cylinders with proteinuria in non-nephrotic range (usually less than 1 g/24 h).2,30
Histopathology
Macroscopic
Multiple small petechial hemorrhages are often present on the surface of the affected kidneys. Sectional cutting reveals small wedge-shaped infarcts and foci of cortical necrosis. These changes are non-specific and can be observed in other microangiopathic thrombotic disorders, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, idiopathic malignant hypertension or in association with some drugs.31
Microscopic
Histological manifestations can vary during the course of the disease and early vascular changes may manifest as excessive intimal myxoid material, thrombosis or fibrinoid necrosis. "Onion skin" lesions develop later, while fibrointimal sclerosis with or without adventitious fibrosis can be the only manifestation of chronic damage or resulting from previous episodes of ARF.32 Acute glomerular changes are often developed as a result of the vascular lesion and the reduction of renal perfusion. Primary glomerular changes are related to glomerular endothelial injury and can manifest as endothelial edema and glomerular capillary thrombosis, although the latter is relatively uncommon.33 In contrast with hemolytic uremic syndrome and thrombotic thrombocytopenic purpura, the primary changes of small vessels predominate over glomerular alterations.34 The thrombi in small vessels outweigh the glomerular thrombi, while the opposite is found in hemolytic uremic syndrome and thrombotic microangiopathy.35
Immunofluorescence
Non-specific staining for immunoglobulin M and C3 can be seen. Fibrinogen staining may be present in glomeruli and arteries associated with fibrinoid necrosis or acute thrombotic microangiopathy.36
Electron microscopy
There is endothelial edema and expansion of the internal rare lamina. Duplication of glomerular basement membrane with cell interposition is present in the chronic stages.36
Prognosis
The use of ACEI has dramatically changed the survival of patients with SRC since before the use of these medications, patients almost always died and only 10% survived the first year.7 A study that included 145 patients treated with an ACE inhibitor found that 89 patients (61%) had good results as defined by not requiring or only requiring temporary dialysis, 55 (38%) did not require dialysis and 34 (23%) required transient dialysis; 56 (38%) patients experienced poor results, 28 (19%) survived on dialysis and 28 (19%) died in the first 6 months.27
The factors associated with a poor outcome are, advanced age, male sex, congestive heart failure, inadequately controlled blood pressure and an initial serum creatinine level greater than 270 mmol/L.37 Exposure to prednisone at the onset of SRC was associated with a significant increase in the risk of death. Hudson et al., estimated a 4% increased risk of death for every milligram of prednisone taken by the patient.38 Timely and appropriate treatment is essential to avoid dialysis and significantly improves the prognosis of these patients.39 The prognosis of normotensive SRC is worse than in hypertensive patients with high mortality rates and renal failure. The absence of high blood pressure is an alert message in renal crisis since it can delay diagnosis and treatment, leading to disease progression.40
Renal transplantation
End-stage renal disease may develop in up to 45-55% of patients even when SRC is recognized and treated quickly.29,41,42 For these patients, renal transplantation is a reasonable option and may offer a survival advantage over chronic dialysis.43 Patients with terminal renal disease due to SRC have worse survival compared to patients with other pathologies. The ANZDATA registry reported that only 10% of these patients were able to interrupt dialysis after an average period of 14 months.43
There have been reports of recurrence of SRC in kidney transplants (estimated at around 20-50%) of cadaveric or living donors, including twins.44 Renal transplantation with allograft can be associated with early onset of renal insufficiency due to SRC and can be announced by some markers of worsening of scleroderma such as progressive thickening of the skin, anemia and cardiac complications.2
Other renal complications in scleroderma
Clinical markers suggesting renal disease, such as proteinuria, elevation of creatinine and arterial hypertension were found in 50% of asymptomatic patients. Renal chronic vascular disease, kidney injury from nephrotoxic drugs (including cyclosporine, and D-penicillamine), oxalate calcium nephropathy, glomerulonephritis and vasculitis associated to anti-neutrophil cytoplasmic antibody have also been found (Table 1).5,13
Calcium oxalate nephropathy
Oxalate nephropathy in SSc is a cause of acute renal tubular injury that is commonly associated with conditions that affect the absorption in the small intestine.45-47 Common pathophysiology requires chronic malabsorption of dietary fats in the small intestine with a relatively conserved colonic absorption. The increase in luminal fatty acids in the small intestine displaces calcium from the oxalate leaving it free to be easily absorbed at the level of the colon. This oxalate is subsequently filtered in the glomerulus and exchanged in the proximal tubules, precipitated, and induces increased inflammation or necrosis of the renal tubular epithelial cells.48-50
The prognosis is poor in patients who develop oxalate nephropathy, however, the duration of renal injury, the extent of intestinal disease, the functional and nutritional status of patients also define clinical outcomes. Early recognition of patients at risk is critical since the establishment of a diet low in oxalate can help prevent this complication. The functions of early use of prokinetic agents, antibiotics for SIBO (small intestinal bacterial overgrowth), calcium and magnesium supplements and probiotics remain to be defined in future prospective studies of patients at risk with systemic sclerosis.45
Isolated arterial hypertension
Patients with SSc also have associated arterial hypertension. Whether the hypertension is a comorbidity or a manifestation of renal vasculopathy is not clear, In a study that included 561 patients with SSc (of which 60% had SSlc) and a mean duration of the disease of 10.9±8.8 years, 23% who had a normal renal function and 43% who had abnormal renal function at baseline, presented hypertension after >2 years of follow-up. In contrast, only 12% of the 675 patients with SSdc in the University of Pittsburgh cohort, who were followed between 1972 and 1993, had hypertension.51 Half of these patients developed hypertension with an average of 6.9 years before being diagnosed with SSc while the remaining patients developed hypertension with an average of 4.7 years after being diagnosed with SSc, however, 61% of these patients received glucocorticoids before the onset of hypertension, suggesting that this treatment might cause hypertension. Therefore, there is no evidence in these studies that hypertension per se is associated with the development of SCR.1
Anti-neutrophil cytoplasmic antibody associated glomerulonephritis in scleroderma
In contrast to SCR, this manifestation develops in patients with long-lasting SSlc. The prevalence is unknown, but approximately 40 cases have been reported in the English language literature.52-57 ANCAS-associated vasculitis (AAV) in the context of SSc is more common in the fifth and sixth decade of life (average age 57, range 19-82) and the male to female ratio is 1:4, similar to the general sex distribution in patients with SSc. The average duration of SSc until the onset of vasculitis associated with ANCAS is approximately 9 years. The majority of patients suffered from the diffuse variant (57%), while about 38% had the limited variant.
It has been postulated that scleroderma vasculopathy exacerbates the interaction of anti-neutrophil cytoplasm antibodies (ANCA) with the endothelium near the vascular pole and the consequent activation of neutrophils in the glomerulus.5
Typically, patients present with crescentic glomerulonephritis, progressive renal failure, mild hypertension and proteinuria. In addition, associated pulmonary hemorrhage has been reported.24
In some cases, the classic reactivity against proteinase-3 or myeloperoxidase is identified, while in others an atypical reactivity of ANCA is demonstrated only by immunofluorescence staining.55 It has been suggested that ANCA tests should be performed in all patients with suspected SRC since vasculitis may be an important differential diagnosis for normotensive SRC.56
Studies on the incidence of ANCA antibodies in not selected patients with SSc have been shown to be 0-12%, but in these patients both MPO and PR3 can be identified by ELISA.57 Up to 13% of patients with SSc were positive for MPO by ELISA, but negative for ANCA by immunofluorescence. The most common clinical manifestation (83%) of AAV was rapidly progressive glomerulonephritis (RPGN), 28% suffered from alveolar hemorrhage, 13% from necrotizing vasculitis that resulted in limb ischemia and 10% from a vasculitic skin rash.57 Similarly to vasculitis associated with ANCA in scleroderma patients, this complication requires therapy with high doses of steroid type methylprednisolone 500-1000 mg intravenous every day for three doses, followed by oral prednisone 1 mg/kg/day (maximum 80 mg), with a progressive taper until suspended in 3-4 months, plus cyclophosphamide at a dose of 15 mg/kg (maximum 1.2 g) every 2 weeks for the first 3 pulses, followed by infusions every 3 weeks for the next 3-6 pulses. In some refractory cases plasmapheresis was added. There are reports of cases that suggest the effectiveness of Rituximab for glomerulonephritis associated with ANCA in scleroderma.57
Isolated proteinuria
Urinary excretion of albumin is generally recognized as a marker of renal vascular damage and a predictor of cardiovascular morbidity-mortality independent of other comorbidities, such as diabetes and hypertension.58 Clinically relevant albuminuria or proteinuria is detected in almost one-third of patients with SSc and indicates not only renal vascular disease, but also systemic vascular disease that is associated with increased morbidity and mortality. Microalbuminuria and tubular proteinuria were present in 50 (45%) of 112 evaluated patients but many of these patients had renal failure, therefore, there was no association between renal function and microscopic proteinuria.29 Additional studies are needed to determine if intervention, for example with ACE Inhibitors, improves outcomes.1
Reduced glomerular filtration rate
A longitudinal Canadian study in more than 400 patients with SSc followed for a median of 2 years with renal function measured by glomerular filtration rate (GFR), observed a slight decrease associated with comorbidities such as hypertension, diabetes mellitus, and comparable to the general population. The reduction of GFR does not appear to be progressive in the majority of patients with SSc and does not predict the onset of SRC.51
Chronic kidney disease in scleroderma
Patients without previous renal crisis may show reduplication of elastic fibers, sclerosed glomeruli, tubular atrophy and interstitial fibrosis, which probably reflects chronic scleroderma changes. Kingdon et al. showed a reduction in GFR in cases of systemic sclerosis without renal crisis.59 Chronic kidney disease, therefore, seems to have a benign prognosis in SSdc. Other causes of renal failure are likely to occur in cases of SSc, including prerenal causes (associated with cardiac and pulmonary arterial involvement) and drugs.29 Although D-penicillamine is rarely used in SSc at present, approximately 20% of patients develop membranous glomeru-lonephritis and proteinuria that improve with medication withdrawal. However, in severe cases, steroids, plasmapheresis and immunosuppression are required. According to the cases reported in the literature, this complication has a mortality rate of approximately 40%.5
Reduced renal functional reserve
A marked reduction in renal functional reserve (RFR) was described in 21 patients with SSc compared to healthy controls (1.9±18.6% versus 34.8±13.9%, p < 0.00).60 In a subsequent study of 28 patients with SSc, 13 of 19 patients with RFR less than 10% at the start of the study experienced a decrease in creatinine clearance greater than 2 ml/min/1.73 m2 per year in 5 years of follow-up, with a final creatinine clearance of less than 70 ml/min/1.73 m2 in 8 patients. Ten patients developed systemic hypertension (grade 1 or 2) and 2 developed albuminuria. No patient with normal baseline RFR (n = 9) developed proteinuria or hypertension.60
Conclusion
Renal disease is an important cause of morbidity and mortality in SSc. While SRC has been one of the most dreaded complications of scleroderma; many different other kidney involvements are possible, including abnormal renal function, proteinuria, renal chronic vascular disease, kidney injury from nephrotoxic drugs, oxalate calcium nephropathy, glomerulonephritis and vasculitis associated to anti-neutrophil cytoplasmic antibody.











 text in
text in