Introduction
Bone health in patients with systemic lupus erythematosus (SLE) is influenced by multiple factors and can lead to different complications including osteoporosis (OP), fragility fractures and osteonecrosis (also named avascular necrosis, AVN). A decline in bone mass translates into a low bone mineral density (BMD) and may lead to osteopenia, OP and eventually fragility fractures. Sub-chondral bone necrosis as a result of insufficient blood supply causes AVN. OP and AVN are prevalent in SLE despite the often-young age of people living with SLE. These complications lead to significant morbidity, mortality, disability, and loss of quality of life. In this paper reviews the epidemiology, pathophysiology, diagnosis, and management of these conditions.
Epidemiology
Osteoporosis. OP may be increased in SLE for many reasons. OP can be divided into postmenopausal, senile and secondary causes such as glucocorticoid-induced osteoporosis (GIOP), metabolic reasons (such as parathyroid dysfunction in SLE patients with renal impairment) and drugs such as heparin and anticonvulsants.
Low BMD values have been consistently reported in the literature among patients with SLE.1,2 In order to pool all the data, Xia et al. did a meta-analysis in 2019 encompassing 70 studies and 33,500 patients. (3 Half of the papers included were published after 2010 so the data may be generalizable to contemporary treatment. The prevalence in SLE of low BMD, osteopenia, and OP was 45% (95% CI 38-51; I 2 96.6%), 38% (95% CI 31-45; I 2 95.2%) and 13% (95% CI 11-16; I 2 90.8%), respectively. Postmenopausal patients had more osteoporosis (21 vs 9%) and less osteopenia (25 vs 42%) compared to pre-menopausal patients. Lumbar spine was found to have more osteoporosis than the hip. The prevalence of osteoporosis increased with age, while low BMD/osteopenia had a U-shaped prevalence over time, with lower BMD in the young and older patients. The risk factors for low BMD were postmenopausal status, non-Afro-Caribbean, higher body mass index, number of births, ever taken prednisolone >10mg/day, and maximal dosage of >50mg/day of oral corticosteroids. The predictors for osteoporosis were menopause, disease duration, and prednisone use. Disease duration, age, higher BMI, history of previous fracture, corticosteroid use, seizures, cerebrovascular events, and increased damage (as measured by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC/ACR DI) were statistically significant risk factors for fractures. Moreover, patients with SLE have shown lower levels of vitamin D compared to the general population, and vitamin D deficiency is common. (4 Vitamin D deficiency is not specific for SLE, as it has been consistently reported in many other autoimmune diseases. (5 Low levels of vitamin D have been associated to low BMD and fractures in SLE patients. A population study of more than 10,000 SLE patients in Korea aged 40 or more years, showed that OP fractures were 3 times higher than age and sex matched controls. (6 In a UK study using an administrative database of 7732 patients with SLE and 28079 matched controls, OP fractures were reported as 2.5 times higher than controls. (7
Men with SLE and low bone mass may be associated with abnormal sex hormones. One small study of 40 men with SLE showed that reduced lumbar spine BMD was associated with age, habitual alcohol use, low BMI, and high doses of GCs. (8
Osteonecrosis. AVN in SLE has variable rates reported. A meta-analysis in 2017 included 58 studies and over 23,000 SLE patients. (9 The AVN prevalence ranged from 1.45% to 33%. Patients were divided into symptomatic and asymptomatic AVN. Remarkably, different diagnostic techniques were used for different locations in the various studies, resulting in a high heterogeneity of AVN frequencies. The pooled prevalence of symptomatic AVN was 8.96% (95% CI 7.37-10.55; I2 93%); the femoral head was the most common location (8.0%, 95% CI 5.88-10.12; I 2 84%). The pooled prevalence of asymptomatic AVN was 28.52% (95% CI 19.46, 37.60, I 2 80%), when multiple locations had been assessed by various imaging techniques such as X-rays, bone scans, and MRIs. According to the largest study published to date, AVN is typically diagnosed 8 years after the diagnosis of SLE, with a median age of 37 years old when AVN is detected and 14% (234 of 1729) had osteonecrosis. (10 The most frequently involved joints were hips and knees, though other joints may also be involved, such as shoulders, elbows, and ankles. The frequency of AVN has been decreasing between 1970 and 2000 and seems to have leveled since then. This may be due to changing epidemiology of SLE over time (perhaps somewhat more mild disease overall over time), less use of long-term high dose corticosteroids and/or less end-stage renal failure.
Many factors have been hypothesized to be related to AVN. In this large longitudinal cohort, (10 no differences were found between AVN cases and matched SLE controls regarding smoking, antiphospholipid syndrome, vasculitis, age, disease duration or immunosuppressive treatment. Only the use of GC reached statistical significance in their multivariate analysis. Additionally, a meta-analysis reviewing the occurrence of AVN in patients with SLE showed that patients who developed AVN were more likely to have a history of GC use (OR 1.79) and current GC use (OR 2.98). Furthermore, patients with AVN had a higher GC cumulative dose than those who did not have AVN (OR 3.71). (11 In Nevskaya's meta-analysis, (9 the following relevant risk factors were identified: African-American ethnicity (OR 1.79), neuropsychiatric SLE (OR 1.99), cutaneous vasculitis (OR 2.08), Raynaud's phenomenon (OR 1.26), Sjogren's syndrome (OR 3.09), arthritis (OR 1.69), serositis (OR 1.8), cytopenia (OR 1.92), and positive IgM anticardiolipin antibody (OR 2.48). Interestingly enough, OP and osteoporotic fractures did not show any significant association with AVN. In terms of treatment, GC use (OR 3.02), especially in pulses (OR 1.95) significantly increased the risk for osteonecrosis. Higher GC cumulative doses (7 g) and maximum daily doses (>10 mg/day) were found in SLE patients with AVN compared to those without AVN. (9 Others have reported that renal disease and steroid-related Cushingoid appearance and hypertension were also risk factors, but the most consistent risk seems to be use of glucocorticoids. (11,12
Pathophysiology
Normal bone remodeling. The bone remodeling mechanism is a balance between bone formation and resorption. The main cells types involved are osteoblasts, osteocytes and osteoclasts. Osteoblasts produce extracellular bone matrix among others. The process results in calcium hydroxyapatite deposition in the bone. This mechanism involves the receptor activator of nuclear factor-KB (RANK) ligand (RANKL), secreted by pre-osteoblasts. This protein can both stimulate osteoblasts and osteoclasts. By binding to osteoprotegerin, RANKL stimulates bone formation by osteoblasts. In contrast, by binding to RANK, the pre-osteoclast lineage differentiates to osteoclasts and bone resorption is stimulated. (13 Osteocytes act as mechanoreceptors for the adaptative response to bone mechanic stress and also play an endocrine role, controlling the bone matrix homeostasis and the calcium and phosphate balance. (14 The whole process can be influenced by different conditions and situations, changing the balance through proinflammatory cytokines or hormones.
Postmenopausal osteoporosis. Estrogen receptors are present in all cell lines in the bone matrix. Moreover, their dysregulation creates a microinflammatory environment that leads to abnormal bone turnover. Estrogen deprivation, mainly occurring after menopause, leads to bone resorption and bone density loss. Oestrogens promote osteoblast differentiation and collagen matrix deposition. They also upstream the RANKL pathway in osteoblasts, downregulating the RANK pathway in osteoclasts. In the absence of estrogens, RANKL expression increases, along with tumor necrosis factor alpha, interleukin (IL) 1n iL-6 and lymphocytes. (13 This situation causes osteoclasts to proliferate and increase bone loss. Finally, the activity of osteocytes is also impaired. In summary, there is an increased trabecular bone resorption for the first few years, subsequently followed by a slower trabecular and cortical bone loss.
Glucocorticoid-induced osteoporosis. Treatment with glucocorticoids (GC), especially at high doses, induces significant bone resorption during the first year of treatment, followed by long term decreased bone formation. (15 GC switch the pre-osteoblast cell differentiation toward adipocyte formation. In addition, osteoblastogenesis is reduced by the overexpression of sclerostin. Furthermore, high doses of GC induce osteoblast apoptosis. GC also increase RANKL production and lower osteoprotegerin. As a result, there is less bone formation, and osteoblasts increase their bone resorptive activity. The effects on osteocytes, with increased apoptosis, alter the bone matrix homeostasis. In the long term, osteoclastogenesis becomes downregulated because of a declining cell population. GC have effects outside of the bone matrix, that can influence bone health. Calcium absorption in the intestines as well as calcium reabsorption in the kidneys is reduced. With GC use, several hormones are altered such as upregulation of parathyroid hormone (PTH) and decreases in insulin growth factor 1 and growth hormone. All these ancillary mechanisms contribute to the loss of bone mass.
Other drugs can cause secondary osteoporosis such as anticonvulsants (carbamazepine and phenytoin), long term use of heparin, long term use of progesterone (injectable medroxyprogesterone acetate for contraception), proton pump inhibitors, aromatase inhibitors, anti-androgen therapy, thyroid over replacement and medication that causes premature ovarian failure (such as cyclophosphamide). (16-19 Other associations of OP in SLE patients can be concomitant hyperthyroidism, celiac disease, inflammatory bowel disease, and SLE related issues such as renal failure, systemic inflammation, and anti-vitamin D antibodies and premature menopause and/or low estrogens and androgens.
Avascular necrosis (AVN)/osteonecrosis. Multiple factors contribute to the development of AVN including genetics, metabolic factors like drugs or toxic substances, and preexisting conditions. (20 SLE and GC use are strongly associated to AVN. (10 During SLE flares, higher levels of homocysteine, antiphospholipid antibodies, increase of tumor necrosis factor, and osteocyte damage and death, may contribute to the disruption of blood flow to the trabecular bone. (21 As a result, bone infarcts would appear prompting necrosis of the bone. After a process of repair with cartilage deposition over the dead bone, the structure collapses resulting in a fracture.
Diagnosis
Osteoporosis. The diagnosis of OP in adults is based on the results of dual-energy X-ray absorptiometry (DXA), a technique to perform bone densitometry (BMD). This is a non-invasive technique, that can be relatively easily performed. There is a strong correlation between the capacity of attenuation of the X-ray of the tissues (bones) and bone strength. Although different areas can be measured for OP detection, currently femoral neck bone density is the preferred site in epidemiological studies22 and the lumbar spine is often used to follow OP treatment. The World Health Organization's definition for OP is a BMD greater than -2.5 standard deviations (SD) of a 30-year old man or woman (T-score), (23 which is generally applicable to people over 65 years old (including postmenopausal women). The use of the Z score (comparison with the BMD of normal healthy people of the same age and sex) can be considered in younger patients. (24 Finally, and out of the scope of this review, childhood OP is defined by the presence of one or more vertebral compression fractures. (25
Avascular necrosis (AVN)/osteonecrosis. The clinical suspicion for AVN is joint pain that may gradually worsen such as in the hip (groin) and it may be associated with decreased range of motion and is often bilateral. Once AVN is suspected, imaging should be done. (26 There is a high heterogeneity in the tests that can be performed to get to this diagnosis, but the most usual ones are plain X-rays (which only detect changes later with collapse of bone and/or secondary joint space narrowing), magnetic resonance imaging (MRI) and bone scans. (9 Based on the most common site for AVN, the hip, Arlet and Ficat established a 4-stage severity classification27: (1) normal hip ox X-ray but diagnosed by MRI, (2) femoral head lucencies or sclerosis by X-ray, (3) a crescent sign within the femoral head indication an advanced stage, and (4) acetabular involvement and space narrowing. Bone scan (bone scintigraphy) had a lower sensitivity than the MRI of affected joint(s) (56 vs 100%) in a study with symptomatic multifocal AVN. (28 An example of AVN is depicted in Fig. 1. Nevertheless, access to plain radiography or bone scans might be more feasible than for MRI, as waiting times and financial costs are significantly higher for the latter. (29
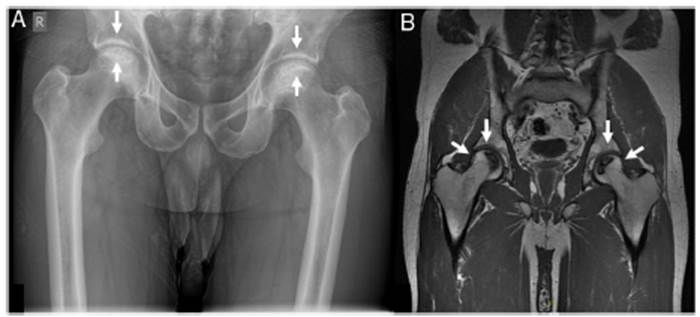
Fig. 1 Bilateral avascular necrosis of the femoral heads in a patient with systemic lupus erythematosus. Panel A shows a pelvic posterior-anterior radiography where subchondral sclerosis is evident at the femoral heads, bilaterally (white arrows). No insufficiency fractures are seen and articular space is preserved in both joints. This X-ray would correspond to a grade 2 according to Arlet and Ficat’s classification. Panel B shows a pelvic magnetic resonance imaging T1 coronal cut with crescentic areas of T1 hypointensity (white arrows) consistent with avascular necrosis involving two thirds of the articular surface bilaterally. There are no insufficiency fractures. This second technique shows a grade 3 AVN as per Arlet and Ficat.
Prevention and screening
Osteoporosis. The finding of a DXA scan suggestive of OP is not the only factor to consider preventing future fractures. As an example, for a similar T-score, patients of different ages can have a hugely different risk for having fractures, as older age has a higher risk of fractures. Additionally, patients with osteopenia (T-score between -1 and -2.5) can also have fragility fractures. Using BMD and the different non-radiological risk factors identified, the FRAX® score was created. (30 This score is able to predict the individualized 10-year risk to develop hip or major OP fractures based on BMD results (which can be missing if there is no possibility to get the test done), age, sex, weight, height, previous fracture, parental hip fracture, current smoking status, GC use, the presence of rheumatoid arthritis, secondary causes of osteoporosis, and alcohol intake. It is limited to patients aged 50 or beyond. FRAX® has been adapted to multiple countries, to match different populations with different risk factors and can be easily accessed online.
Reviewing the guidelines indications for OP prevention and screening, the 2019 update of the EULAR recommendations for the management of SLE does not address this topic. Another recent (2018) practice guideline for adult patients with SLE from the Canadian Rheumatology Association, (29 had low level evidence to make the recommendations for bone health in SLE. These indications were the same as the general population, (31 as specific information for SLE patients was scarce. The recommendations were:
Use the FRAX® score for patients ≥50 years old.
All individuals should get a baseline BMD if age ≥65 or for menopausal women and men aged 50-64 if they have any clinical risk factor (such as fragility fracture after age of 40, prolonged use of GC ≥ 7.5 mg/day for more than 3 months, use of aromatase inhibitors or androgen deprivation therapy, parental hip fracture, vertebral fracture or osteopenia on radiographs, current smoking, high alcohol intake, weight <60 kg or weight loss >25% of adult weight at age 25 years, concomitant rheumatoid arthritis)
For adults <50 years old, BMD is indicated if fragility fractures are present, prolonged use of GC >7.5mg/day for more than 3 months, use of aromatase inhibitors or androgen deprivation therapy, hypogonadism or premature menopause before the age of 45, malabsorption syndromes (such as celiac or inflammatory bowel disease), primary hyperparathyroidism, or other disorders associated with rapid bone loss and/or fractures.
Risk reassessment including history and physical exam, as well as a BMD scan in patients with risk factors should be repeated periodically if high risk (such as every 1-3 years). Patients >50 years with no risk factors with a 10-year fracture risk <10% should be reassessed every 5 years. No data are available for younger patients.
Screening for 25 hydroxy vitamin D deficiency should be considered for OP assessment.
In the specific setting of GC-induced OP, the ACR published a guidance document in 2017. (32 The different suggestions for fracture risk assessment are similar to the abovementioned Canadian ones:
Clinical risk factor assessment should be done within the 6 months after starting GC treatment.
For adults <40 years of age with risk factors (history of OP fracture, or Z-score < -3 at hip or spine, or >10%/year loss of BMD at hip or spine, or GC ≥ 30 mg/day, or other OP risk factors) baseline and every 2-3 years BMD testing is warranted despite treatment.
For adults ≥40 years of age BMD and the FRAX® tool should be used every 1-3 years if OP treatment naïve. For patient with ongoing OP treatment, in case of GC ≥ 30 mg/day, or OP fracture >18 months before starting OP treatment, or poor medication adherence/absorption, or other OP risk factors, BMD testing every 2-3 years. Finally, the patients who completed OP treatment should have BMD testing every 2-3 years.
Osteonecrosis. Some studies have used radiographs to screen for asymptomatic AVN systematically. There is no clear evidence showing that all asymptomatic cases will progress to clinically relevant AVN. Finally, there is no validated screening tool for AVN. Hence, no specific recommendation can be given and prevention should be based on avoiding risk factors, especially moderating the exposure to GC. (10,29 However, if AVN is suspected, a reasonable algorithm could be to X-ray the affected joint(s), perform a MRI if the radiograph is normal or not pathognomonic and a bone scan only if MRI is not available. (33 For confirmation of the diagnosis, an MRI is better to detect the extent of AVN within a joint than the bone scan. So, bone scans may be eliminated from the diagnostic algorithm in many centers.
Treatment
Osteoporosis
Non-pharmacological treatment. Some risk factors for OP are modifiable. Smoking cessation and a limited alcohol intake may help to reduce the decrease of BMD and the incidence of secondary fractures. Doing some weight bearing exercise is also recommended. (34 Vitamin D supplementation (800 IU/day) has been associated with a reduction of 15-20% of non-vertebral fractures. (35 Some recommendations are even higher (such as 1000 to 2000 IU/day). (36 Oral calcium intake or supplements between 700 and 1200 mg/day are advisable, but calcium on its own has failed to reduce fracture risk. (37 Additionally, many recommendations are a total of 1000 mg a day of calcium intake using dietary calcium and only supplementing additional calcium to total 1000 mg as there was thought to be a risk of coronary and/or aortic calcification above 1000 mg/day38,39 but this seems not to be consistent in several studies. (40
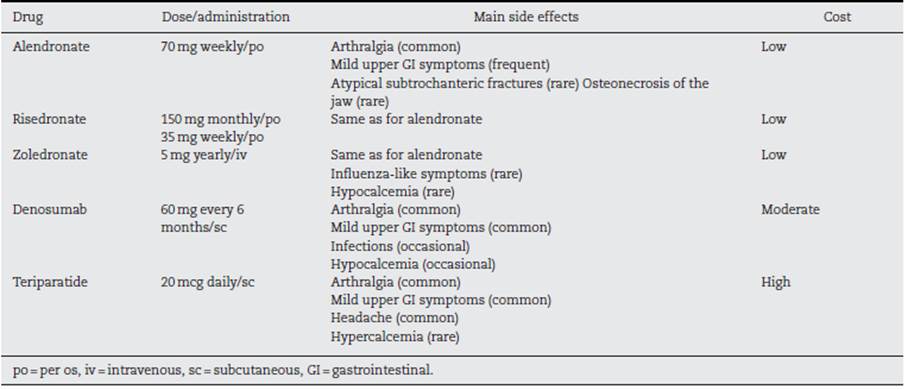
Pharmacological treatment. The indications for OP treatment in SLE patients are active treatment of documented OP or prophylaxis in the context of long-term GC treatment. The main drugs available for the treatment of OP are bisphosphonates (alendronate, risedronate, pamidronate, zoledronate), denosumab (rank ligand inhibitor), parathyroid hormone agonists like teriparatide, and romosozumab, new antisclerostin monoclonal antibody and less used are hormonal treatment and raloxifene. Most of the evidence comes from studies in postmenopausal women without SLE. Access to drugs and drug reimbursement may be an issue, which can vary geographically. In a systematic review41 comparing the efficacy of different OP treatments to prevent fractures all the drugs studied (alendronate, ibandronate, risedronate, zoledronic acid, denosumab, teriparatide and raloxifene) prevented vertebral fractures in women with OP (number needed to treat was between 60 and 89 to prevent 1 fracture over 1-3 years of treatment). Only a study showed zoledronic acid preventing vertebral fractures in men (most studies are in women) with OP (number needed to treat 30 to prevent 1 fracture over 2 years of treatment). Regarding non-vertebral fractures, alendronate, risedronate, zoledronic acid, denosumab and teriparatide, prevented these fractures in women with OP (number needed to treat 50-60 to prevent 1 fracture over 1-3 years of treatment). Needless to say, the NNT is higher if osteopenia is treated without a fragility fracture. The side effects differ between the different drugs. Bisphosphonates can cause mild upper gastrointestinal (GI) symptoms and rarely atypical sub-trochanteric fractures and osteonecrosis of the jaw. Zoledronic acid can cause hypocalcemia and influenza-like symptoms. Denosumab can cause mild upper GI symptoms, and hypocalcemia. In patients treated with teriparatide, mild upper GI symptoms, headache and hypercalcemia have been reported. Since raloxifene (and postmenopausal hormonal treatments) increases the risk for thrombosis, their use in SLE patients with potential antiphospholipid antibodies should likely be avoided. Strontium is not approved in most countries for the treatment of OP.
British34 and Canadian31 OP guidelines agree in recommending oral bisphosphonates as the most cost-effective measure, followed by denosumab. Side effects and comorbidities can determine the chosen initial therapy (Table 1). The possibility of using teriparatide is restricted to third line due to its high financial burden, but it is highly effective activating osteoblasts more than osteoclasts and thus increasing bone mass. Treatment duration is usually for a maximum of 2 years. BMD may decline slightly in patients treated with bisphosphonates after stopping them. (42 However, those treated with denosumab will have a fast and significant drop of their BMD, and an increased risk for new fractures after treatment cessation. (43
For prevention of GIOP, trying to avoid or reduce GCs in SLE should be considered. Keeping doses below 7.5 mg/day or using IV pulses instead of higher regular oral doses of GC, along with an earlier initiation of immunosuppressive drugs can reduce the incidence of GC related side effects, including GIOP. (44 When starting a patient on high doses of GC, the BMD decreases quickly in the first few months especially trabecular bone which is more at the lumbar spine than the hip. This bone density loss is partially. (45 In terms of focused pharmacological measures for GIOP, the latest recommendations from the ACR in 2017 suggest:
Same non-pharmacological interventions as in OP.
For adults <40 years of age with risk factors (history of OP fracture, or Z-score<-3 at hip or spine and prednisone ≥ 7.5 mg/day, or >10%/year loss of BMD at hip or spine, or GC ≥ 30 mg/day, or other OP risk factors) start with oral bisphosphonates. If not possible, consider the other medications used in OP.
For adults ≥40 years of age with risk factors (history of OP fracture, or men ≥50 years and premenopausal women with a T-score ≤ -2.5 at the hip or spine, or FRAX (adjusted for GC) 10-year risk for major OP fracture ≥ 10%, or FRAX (adjusted for GC) 10-year risk for hip fracture >1%, or prednisone ≥30mg/day; the recommendation is oral bisphosphonates. In case of intolerance, by order of preference: intravenous zoledronic acid, teriparatide, and denosumab.
In women with childbearing potential, oral bisphosphonate would be the first line and teriparatide the second. In the event of a pregnancy, the patient should be advised that there is not enough information to consider them safe.
There is very little data about using OP treatment in patients below the age of 40, so closely monitoring SLE patients on GC and supplementing with calcium and Vitamin D are often initially tried. Recently, a retrospective study of 203 SLE patients demonstrated that approximately 60% of SLE patients at risk of GIOP received Vitamin D and in those over the age of 40 years, 70% who should have been on bisphosphonates were not prescribed with them; whereas 10% were prescribed bisphosphonates but did not meet the ACR 2017 GIOP guidelines. (46 Thus, there is a gap in care.
Osteonecrosis. The intention to preserve the joints will guide AVN treatment. Patients with stage 1 AVN can spontaneously regress. Observation can be an acceptable option, especially in areas with less weigh bearing, like the shoulders. Hips and knees have a greater likelihood to progress to symptomatic AVN. Once the damage is established, bone preserving strategies should be pursued such as low impact exercises, and symptomatic treatment with paracetamol, or NSAIDs if not contraindicated. It is uncertain if bisphosphonates are helpful. Once the joint is damaged, there is likely less chance of worsening the joint by giving symptomatic intra-articular corticosteroid injections. However, there is no data to support the latter approach but it may improve pain and prolong the time for a joint replacement. Finally, joint replacement is reserved to patients with joint collapse. (47 Unfortunately, no SLE guideline addresses AVN treatment.
Pharmacologic treatment. Multiple interventions have been tried, but only in small observational studies and there are no randomized trials. Alendronate at a dose of 10 mg daily, showed a hip survival (no need of joint replacement) after 3 years in 87% of patients with hip AVN. (48 Other agents such as lipid lowering agents and anticoagulants have been reported with some potential benefit47 but are not the standard of care.
Physical modalities. Extracorporeal shockwave therapy, hyperbaric oxygen, and pulsed electro-magnetic therapy have shown some results, but more extensive studies are warranted to provide guidance. (47 Again, these are not standard of care.
Surgical treatment. Bone preserving options include core decompression with or without bone grafting, percutaneous drilling, bone grafting with vascularized or non-vascularized grafts, and osteotomy. (26 Bone decompression basically consists in drilling some holes into the necrosed subscortical areas of the bone. The technique has evolved from drilling bigger holes to currently smaller ones, preventing about 70% of the hips of being replaced. (49,50 A comparable success rate of 87% has been reported for knee osteonecrosis in SLE patients. (51 Free vascularized fibular grafting (FVFG) is a more complex surgical technique, which can make lead to donor site complications and a longer operating time. In a study with 80 hips from SLE patients with AVN, after 4.3 years of mean follow up, no hips needed arthroplasty. (52 Finally, osteotomies like trans-trochanteric anterior rotational osteotomy can be considered, but studies in SLE patients showed a 50% rate of reconversion to arthroplasty. (53 At most centers none of these options are routinely performed. Joint replacements are the treatment of choice for end stage AVN.
Arthroplasty. This is the preferred treatment once there is a collapse of the necrosed bone. There is significant positive experience in patients with SLE and hip AVN, with success rates ranging from 90 to 100% and considerable functional improvement. (54,55 There are no differences in outcomes between SLE and non-SLE patients. Other joints such as knees and shoulders can be replaced successfully. (47 The downside of total joint replacements in young patients with SLE and severe AVN are that there maybe a need for revisions or repeat arthroplasties over their lifetime but this is not a contraindication for performing surgery and this can greatly improve quality of life including joint pain and function.
Conclusions
Problems related to bone health (OP and AVN) are common in SLE patients and lead to significant functional impairment and loss of quality of life. Menopause, disease duration and prednisone use are predictors for OP. Multiple risk factors are related with OP fractures including disease duration, age, higher BMI, history of previous fracture, corticosteroid use, seizures, cerebrovascular events, and higher damage. The rationalization of the use of glucocorticoids will have an impact both on osteoporosis and low bone density as well as in osteonecrosis. Screening with bone densitometry when appropriate and early pharmacological treatment will reduce complications in osteoporosis. AVN is strongly related to glucocorticoid use and no screening is recommended. Avoiding steroids should prevent AVN in most people with SLE and end stage AVN is treated with a joint arthroplasty often with very good results.