Methodology
A structured search of the literature was carried out, where two of the authors (KOU and DAMA) used the PubMed, Google Scholar, LILACS and Cochrane databases, in order to identify the largest number of studies that correlated aspects of the environment and SLE, initially in Colombia and Latin America and subsequently around the world. The MeSH terms used were: Environment, Social Determinants, Lifestyle, Hormones, Pollution, Occupational Exposure, Occupational Environment, Systemic Lupus Erythematosus. The authors selected the most relevant articles that were then grouped according to the type of risk factor; finally the groups that included studies in humans and had largest and the best evidence were selected.
Introduction
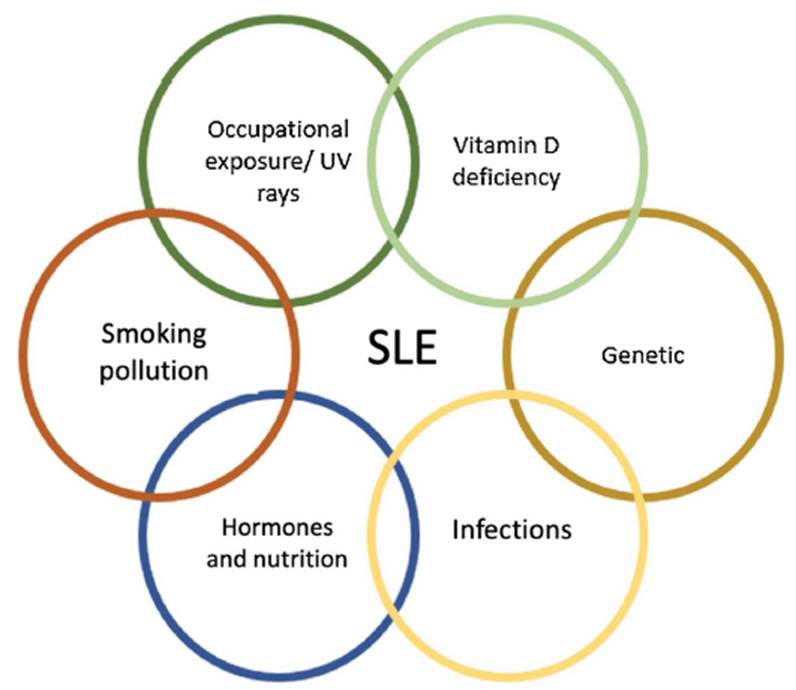
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease influenced by genetic, sociodemographic, and infectious factors. It is estimated that the adjusted prevalence for women and men is 204.3 and 20.2 per 100,000 (respectively) in Colombia, predominantly affecting the population between 45 and 49 years old, with a female: male ratio of 7.9: 1.1 Genomic studies have found polymorphisms in different nucleotides involved in the pathophysiology of SLE; however, the individuality of these mutations fails to explain all the manifestations of this disease or its development. This is why a multifactorial model that includes genetic aspects influenced by family history, exposure to silica, (2 alcohol, (3 exogenous estrogens, (4 smoking, (5 and infections 6 may provide a more comprehensive explanation of the development of SLE as well as some of its manifestations (Figure 1). This review addresses the up-to-date evidence of some of the environmental factors and the risk of developing SLE.
Genetics, Race and Ethnicity
SLE is influenced by multiple environmental and genetic factors. It has a wide spectrum of clinical presentations, which are influenced by the mutation of several genes. In Latin America, for example, the manifestations of late onset lupus (over 50 years) are different from young-onset lupus; i.e., older patients have less SLICC criteria, renal compromise and use of cyclophosphamide. (7 Polymorphisms in the genes that encode regions of the MHC (Major Histocompatibility Complex) represent one of the most important risk factors for the development of SLE, especially HLA-DRB1 and HLA-DQB1. (8-10 A meta-analysis conducted in a Latin American population, which included Colombian patients, showed the association of HLA-DRB1 * 0301 and SLE as a risk factor and the protective effect of HLA-DR5 specifically with the presence of the DRB1 * 1101 allele. (10 P. Guarnizo-Zuccardi et al. explored the association between polymorphisms of TNF alpha, TGF beta, IL 10 and IL 6 and the susceptibility for the development of SLE and found that the single base polymorphisms in the TNFa 2308 allele and in the codon 25 of TGFb1 are associated with SLE in the Colombian population, (11 a finding also reported by Correa Pa et. al, but this was not reproducible in German or Mexican populations. (12
Signal transducers and activators of transcription (STATs) regulate the hematopoietic process through the binding of cytokines to a cell surface receptor; mutations in some regulators of these processes have been related to the development of SLE and rheumatoid arthritis; TJ Palomino-Morales et. al.13 found in a Colombian cohort, a significant influence of the STAT4 rs7574865 allele on the development of SLE (P < 0.001; OR 1.62, 95% CI 1.22-2.16) and at a higher frequency as compared with North Americans 14 and Koreans. (15
Ethnicity is a social and biological composite which is influenced by cultural, geographical, and socioeconomic aspects. (16,17 In contrast, race refers to a genetically homogeneous group. (18 SLE has been shown to present clinically in a heterogeneous way, with more severe, acute and frequent manifestations in younger people among Hispanics, African descent individuals and Asians. (18 An example of this are the studies conducted in Medellín where it has been observed that lupus nephritis is an early presentation compared to what has been reported in other countries. The study by Tatiana Méndez Rayo et. al.19 evidenced that the presence of anti-C1q in Colombian patients is much more frequent in patients with lupus nephritis. (20 On the contrary, in Mexico, Simón et. al. correlated anti-nucleosome antibodies with proteinuria, which exemplifies the variability among countries, races and ethnicities. (21
Infections
The effectiveness of the immune response depends on the ability to differentiate self from foreign and develop a strong enough response to eliminate a microorganism. This ability of the immune system can be altered by host-associated factors (genetics, race and ethnicity), as well as external environmental factors, which promote the production of autoantibodies and therefore the development of autoimmune diseases including SLE.
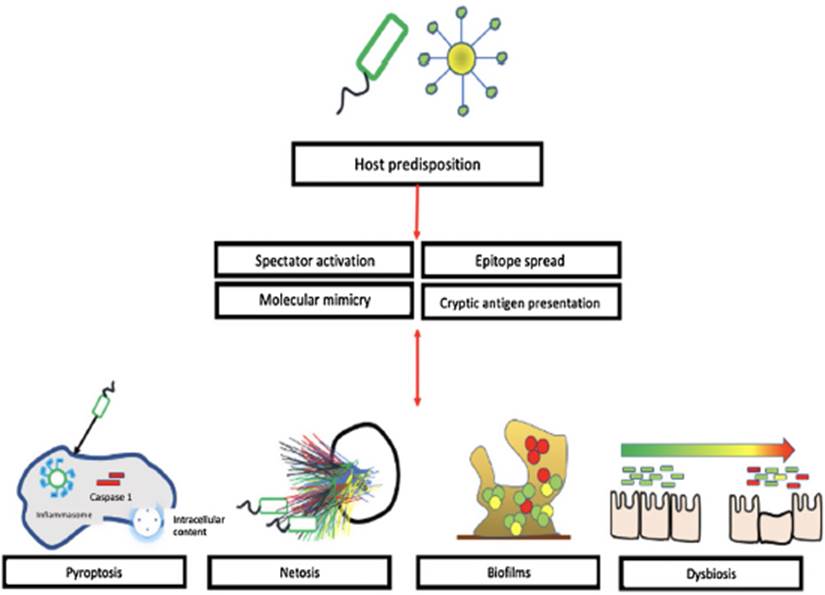
In the pathophysiology of SLE, autoantibodies against nucleic acids and binding proteins play an important role in the development of the disease. These antigens can present as pathogen-associated molecular patterns (PAMPs) of viruses or bacteria and consequently be recognized by pattern recognition receptors (PRRs), as is the case with double-stranded RNA and DNA, which are recognized by toll-type receptors (TLR) seven and nine. (22 Bacterial genetic material can be exposed by various mechanisms including Biofilms, NETosis and pyroptosis, among others. (22 Battaglia et Al. found significant changes in the bacterial microbiome of lupus patients as compared to control subjects; (23 these findings suggest a relationship between dysbiosis and the development of SLE.
Invasive bacterial infections are more common and often more severe in lupus patients; these include infections caused by Staphylococcus aureus, Salmonella enterica, Escherichia coli, Streptococcus pneumoniae and mycobacteria, These bacterial infections have been found to trigger the disease, through increased immune activation and inflammation. (23 This interaction is part of the foundation of the autoimmunity theories related to environmental infections such as molecular mimicry, epitope propagation, activation of bystanders and presentation of cryptic antigens. (24,25 Figure 2 illustrates the theories that relate environmental infection with the development of SLE.
The material contained in the nucleus of bacteria and host cells is one of the main autoantigens associated with SLE. Neutrophils are the first line of defense against microorganisms, they use phagocytosis, reactive oxygen species (ROS), enzymes, and the release of chromatin fibers or extracellular neutrophil traps (NET) to eliminate microorganisms. NETosis is a benign defense mechanism that promotes the arrest of infection and the activation of the inflammatory response; however, in predisposed hosts it can trigger the production of antibodies against auto proteins that can be detected by dendritic cells and lead to inappropriate activation of the immune system. For example, NETosis products have been identified in the skin, kidneys, and blood of patients with SLE. (26 Additionally, research has shown that the absence of enzymes that degrade NET components is also related to the onset of the disease in animal models; this is the foundation to assertain that it is not just the exposure of genetic material, but also the amount and the duration of the exposure which may be associated with the SLE; a case in point is DNase I, since the absence of DNase has been associated with the exposure of self-antigens and the subsequent development of lupus nephritis. (27
Pyroptosis is another form of programmed cell death that depends on the activation of caspase 1. Initially it was believed that it was typical of antigen-presenting cells; however it has been shown to be present in neurons, hepatocytes, hematopoietic stem cells, among others. This pathway is activated by contact between microorganisms or harmful molecules and the NLRs (NOD-type Receptors), leading to the activation of the inflammasome, caspase 1 and the release of primarily IL-1b and IL-18. In addition to releasing inflammatory proteins, this process has been linked to the exposure of intracellular material to macrophages and neutrophils; the cells responsible for pyroptosis must be eliminated without producing an inflammatory response through the expression of molecules such as ATP and phosphatidylserine that mediate the DAMP signals of "find me" and "eatme".28 Biofilm-producing bacteria in the oral cavity have been linked to high levels of anti-dsDNA and increased SLE activity. (29
One of the most studied infections is EBV. Studies evaluating the presence of EBV infection in SLE versus healthy subjects have found that the viral load of EBV is 10 times higher in blood cells, and particularly in lymphocytes, (30-32 suggesting a relationship between the lytic activity of the virus and SLE. This relationship could be mediated by the RNA / SSB complex that has the ability to activate TLR 3 and molecular mimicry mediated by EBV nuclear antigen-1 and lupus antigens. (33 Although there is a pathophysiological substrate between EBV infection and SLE, this analogy is difficult to demonstrate clinically given the high prevalence of the infection in the population. A meta-analysis of 25 case-control studies assessed the prevalence of anti-EBV antibodies in SLE patients compared to healthy controls. There was a higher prevalence of IgG anti capsular antigen, IgG anti-EA-D and anti IgA-VCA in patients with SLE versus healthy controls (OR 2.08 (95% CI 1.15 - 3.76), OR 4.5 (95% CI 3.00-11.06) and OR 5.05 (95% CI 1.95-13.13) respectively), but not for anti-EBNA1 (OR 1.45 (95% CI 0.7-2.98)). (34
Parvovirus B19 (PV-B19) infection has been associated with an increased risk of SLE reactivation. A study conducted by Guillermo Valencia Pacheco et. al. 35 assessed the correlation between PV-B19 infection in Mayan women in Mexico and the development or activation of SLE; they found that the presence of IgM, IgG and the high viral load of the PV-B19 could be a trigger for SLE. Cutaneous leishmaniasis associated with antiphospholipid syndrome has been reported as a strange correlation in Colombia. Ariel Herrera P et. al. 36 described this dual presentation in a 24-year-old Colombian patient, not ruling out that the infection could have triggered the immune pathology, as previously described by Santiago M et.al. and the Kala-azar syndrome. (37
Hormonal Influence
Women of reproductive age are the group most affected by SLE. (38 The relationship between hormones and SLE does not only involve the dysregulation of the homeostasis of a single hormone, but rather a number of alterations in different pathways that give rise to an increased risk of developing SLE. In the meta-analysis published by McMurray RW et. al. 38 high levels of estradiol and procalcitonin, as well as low levels of testosterone and dehydroepiandrosterone are correlated with the presence of SLE compared to control women.
Estrogens act in multiple ways on B cells. They increase the expression of bcl-2, interfere with naive B cells immunotole rance, promote B cells resistance to apoptosis, and contribute to the loss of tolerance of DNA-reactive B lymphocytes. (39-41 All of this happens through both genomic and non-genomic pathways; the genomic pathway is classified as classical and non-classical. In the classical pathway, the ERα and ERβ receptors in the cytoplasm, after binding to estrogens, undergo a conformational shift that allows them to move to the nucleus and join the target gene to regulate the gene response. In the non-classical pathway, estrogen binds to its receptor which can interact with other transcription factors or can be activated by cofactors such as specificity protein 1 (Sp1), activator protein 1 (AP-1), NF-kB proteins and p300. (42 These receptors can be found in reproductive cells, and in the immune system, so that the activation, inhibition or mutation of any of the regulators of these pathways will lead to an altered immune response. (43 In oophorectomized mice treated with propyl pyrazole triol, an ERα receptor agonist, there was an increase in proteinuria and autoantibody levels, indicating a pro-inflammatory role of ERα which has not been demonstrated with ERβ. (44 Likewise, the frequent administration of estradiol in models without ERα induces a predominantly Th2 response, consequently increasing kidney damage in mice. (45 Moreover, the down regulation of the ERK signaling pathway and DNA hypomethylation have been correlated with SLE; estrogens through the suppression of the ERK pathway by phosphorylation, stimulate T cells and promote the development of the disease. (46
Interleukin 6 (IL6) plays a key role in SLE-mediated damage. The differentiation from naive LTCD4 to Th17 is mediated by IL6 and therefore alters the Th17 / Treg ratio. This cytokine inhibits the differentiation of Tregs by blocking the TGFβ-mediated effect. This would explain why elevated IL6 urine and blood levels are correlated with increased disease activity. Estrogens induce the production of IL6 in mice and humans, which is consistent with the study by Olivieri F et.al 47 where women and older people had higher levels of IL6.
Regarding stable disease and family planning, Petri et al. 48 in a non-inferiority, randomized, double-blind trial, prospectively evaluated the effect of oral contraceptives on lupus activity in premenopausal women with systemic lupus erythematosus. The trial population included 183 women with inactive or stable active SLE from 15 US clinics, excluding women with high or moderate levels of antiphospholipid antibodies and / or a history of thrombosis. No significant differences were found in clinical reactivation between the group treated with oral contraceptives vs placebo; Sánchez et al. 49 compared oral contraception with conjugated estrogens, only progestogens, and an intrauterine device, and after one year of follow-up, there was no difference in terms of disease activity using the SLEDAI index; however, the patients receiving estrogen therapy were associated with thrombosis. The conclusion is then that oral contraceptives can be safe in patients with stable SLE, but there may safety concerns in patients with antiphospholipid antibodies and the development thrombotic episodes.
Ultraviolet radiation
Ultraviolet (UV) radiation is a short, high-energy wave that is part of the spectrum of optical radiation. (50 There are three types of UV radiation: UV-A (320 to 400 nm wavelength range), UV-B (290-320 nm wavelength range) and UV-C (200 to 300 nm wavelength range 290 nm); (51 both UV-A and UV-B rays are biologically associated with immunosuppression states, UVA rays represent more than 95% of the total UV radiation that reaches the earth's surface, (52 but it is not absorbed by the atmosphere and has little uptake by proteins and nucleic acids, unlike UV-B rays that are strongly associated with the development of skin cancer, sunburn, and autoimmune states. (53
The mechanism whereby sun exposure plays a role in the pathogenesis of SLE is still being investigated; however it is known that both UV-A and UV-B rays can exert an indirect effect on the deep layers of the skin, causing DNA damage via the production of reactive oxygen species. (54 These changes result in the production of new forms of self-antigens and self-reactive T cells which, in individuals with some predisposition, can trigger the disease if there is constant exposure of these antigens. (55 A recent study showed that UV-B rays reduce the level of DNA methylation of CD4 + T cells in SLE patients, both with active and inactive disease, as compared to controls (p = 0.002); this may account for the significant association of epigenetic changes to the development and progression of SLE. (56
Although low levels of vitamin D are common in our population an there is extensive literature describing the relationsnip between vitamin-D deficiency and autoimmune diseases, there are still some gaps in our knowledge regarding treatment efficiency, dosing, optimal vitamin-D levels and the effectiveness of UV rays exposure. About this last point, sun exposure in non-SLE patients does not significantly increase vitamin-D serum levels, but may increase the risk of adverse effects related to UV exposure, including triggering lupus flares.
Vitamin D
Since the discovery of the relationship between vitamin D and bone metabolism in 1922, a lot of research has been done to expand our knowledge about the relevant role of vitamin D in human physiology. It is also interesting to highlight the relationship between the development of SLE and vitamin D deficiency. (56
Vitamin D receptors (VDR) have been detected in almost all human tissues and are present in every cell of the immune system. The data suggest that VDR expression is necessary for the development of two types of cells: NK cells and CD8αα T cells, which inhibit autoimmunity. (57 With regards to the activation of T lymphocytes, vitamin D modulates cytokine production, inhibiting pro-inflammatory cytokines (IL-2, interferon-γ, TNFα, IL-9, IL-22) and promoting the production of anti-inflammatory cytokines (IL- 3, IL-4, IL-5, IL-10). (56
In a study published by Cardona et al. (57, in a small sample of 51 patients from an intermediate city in Colombia, an inversely proportional relationship was found between the levels of 25-hydroxy vitamin D and the severity of the disease activity measured with SLEDAI 2K, with higher scores in patients with late-onset lupus, polyautoimunity and use of corticosteroids. Petri et.al monitored 1006 patients over 128 weeks. SLE patients with 25-hydroxy vitamin D <40 ng/mL were supplemented and a 20 unit increase in 25-hydroxy vitamin D was associated with a mean decrease of 0.22 (P = 0.032) in the SELENA-SLEDAI index. The urine protein to creatinine ratio decreased by 2% (p = 0.0009). (58
It is well established that ultraviolet light exposure is the main source of vitamin D; however, as previously mentioned, exposure to solar radiation is associated with the development and progression of SLE. Further studies are needed on the direct relationship of this hormone with the pathophysiology of SLE, and additional research on controlled sun exposure and early supplementation to avoid vitamin-D deficiency. Another relevant consideration is that low levels of vitamin D may be the consequence of a chronic disease. (59
Air pollution and cigarette smoking
There is the hypothesis that air pollution is associated with the development of systemic rheumatic diseases, including SLE. (60 Particulate matter is an important component of pollution and one of the six air pollutants according to the Environmental Protection Agency (EPA). The particles contributed through vehicle exhaust pollution have been associated with inflammatory changes and with autoimmune diseases. The prevalence of SLE in urban areas with larger number of motor vehicles is strikingly higher as compared to rural areas; Bernatsky et.al 59 conducted a study in Canadian population and found that exposure to microparticles may be associated with an increased risk of systemic autoimmune rheumatic diseases. The exact mechanism of action is not well understood; however, the formation of reactive oxygen species (ROS) appears to be an important factor. (50
On the other hand, the prominent role played by cigarette smoking in the development of neoplasms and cardiovascular disease is all is well known; however, its role with regards to the immune system should not be ignored, since smoking increases the production of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, GM-CSF and decreases the levels of anti-inflammatory cytokines such as IL-10. (45,50 A meta-analysis revealed a small but statistically significant association between active smokers and the risk of SLE (OR 1.5, 95% CI 1.09 to 2.08), though this relationship that was not observed in patients who stopped smoking.1
The biological pathway through which cigarette-smoking increases the prevalence of autoimmune diseases is not yet fully characterized; however, several potential mechanisms have been suggested, including the ability of cigarette smoke to release intracellular antigens through tissue hypoxia or toxin-mediated cell necrosis, precipitating an immune reaction in susceptible individuals. (61
Smoking influences the number and function of peripheral blood lymphocytes (PBL) through a process not well understood yet. Bijl et al62 conducted a study to determine whether the immune impairment caused by smoking could be related to changes in the expression or functionality of Fas, a cell surface molecule that plays a central role in immune homeostasis and cytotoxic activity. The study found that smoking is associated with a higher expression of Fas in PBL in general, and in B cells in particular, which could make these cells more susceptible to apoptosis.
There is also evidence showing that the components of cigarette smoke such as nicotine, tar, free radicals, among others, are involved with the pathogenesis of SLE through oxidative stress and directly damaging the cell DNA by upregulating genes and driving specific genetic mutations. (50 Barbhaiya et al63 conducted a study including 286 cases of SLE in which they evidenced that active smokers had increased dsDNA+ SLE risk (HR 1.86 [1.14-3, 04]), while past smokers did not (HR 1.31 [0.85-2.00]).
It has been found that active smokers have significantly higher SLE activity, poorer quality of life, more severe skin involvement, more episodes of pleurisy and peritonitis and express more neuropsychiatric symptoms than past smokers and those who never smoked. (64,65 Lupus nephritis does not appear to be related to cigarette exposure; (66 however, smoking at the onset of nephritis was strongly associated with differences in time to the development of end-stage renal failure, and is an important potentially modifiable risk factor that can influence the prognosis of the disease. (67
Exposure to silica
People who have had some exposure to silica with or without silicosis are at increased risk of developing autoimmune diseases. (68,69 In a prospective study, 95 cases of silicosis were studied, finding that a total of 54 (11.0%) of the patients had concomitant systemic autoimmune disease. (70 Silica particles have been shown to mediate the release of pro-inflammatory cytokines, alter T cell stimulation, and decrease regulatory T cells. In mice predisposed to developing SLE that were exposed to silica crystals, it was possible to demonstrate that contact with these crystals correlated with lung inflammation, IgG and C3 deposits in the kidney, as well as the formation of autoantibodies. (71 Similarly, the concomitant presence of lymphocytic choriomeningitis virus and silica crystals in a murine model evidenced that exposure to these factors triggered SLE-associated autoantibodies such anti chromatin, RNP and Sm antibodies. (72
Studies in Europe and in the United States, in both urban and rural populations, have shown a relationship between SLE and exposure to silica. (73,74 In Latin America, Michelle C Rocha et al. 75 gathered 70 Brazilian workers exposed to silica and studied the expression and polymorphisms of cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD- 1). They found a decreased PD-1 expression, which is essential for maintaining T Lymphocyte tolerance. Their findings are also consistent with the findings described in North American and European populations, where silica is related to lymphopenia at the expense of decreasing CD4 T lymphocytes, which has been associated with the development of SLE. (76
Conclusions
The knowledge about the risk factors and triggers of SLE helps in our understanding of the pathophysiology of the disease and could lead to the development of strategies for early management and control, particularly focusing on the modifiable risk factors. However, assessing exposure to some environmental factors may prove to be challenging, since throughout their lifetime, consciously or unconsciously, people are exposed to those factors but in order to establish the relationship between risk factors and actual disease, those factors must be quantitatively measured. At the Colombian and Latin American level, there are not enough studies on environmental factors and their relationship with SLE; further research should be encouraged on specific local subgroups in order to identify risk factors and to develop management and prevention guidelines applicable to our population.