Introduction
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disorder characterized by the presence of a wide range of serum autoantibodies such as antinuclear (ANA) and anti-double-stranded deoxyribonucleic acid (anti-dsDNA) antibodies. Furthermore, the serum of patients with SLE exhibits antineutrophil cytoplasmic antibodies (ANCAs).1) ANCAs are a group of autoantibodies directed against proteins predominantly expressed in the cytoplasmic granules of neutrophils. Small vessels are the usual target of accumulating ANCA autoantibodies, which can affect any organ in the body in patients with ANCA-associated vasculitis.
Two main laboratory techniques are currently used to detect ANCAs. The standard method for ANCA detection in the serum is indirect immunofluorescence (IIF) of ethanol-fixed human neutrophils smeared onto glass slides. Other fixation methods (e.g., formalin) can also be used. Classical patterns evidenced in neutrophil IIF are cytoplasmic fluorescence with central interlobular accentuation (c-ANCA), which usually occurs with proteinase 3 (PR3)-ANCA specificity, and perinuclear fluorescence often with a nuclear extension (p-ANCA), which frequently occurs with myeloperoxidase (MPO)-ANCA specificity. (2 Additionally, atypical ANCA patterns can be detected in ethanol-fixed neutrophils in the presence of ANA and other autoantibodies against several antigens (such as lactoferrin, lysozyme, azurocidin, elastase, cathepsin G, bactericidal/permeability-increasing enzyme, among others) present in the cytoplasmic granules of the neutrophils. (3,4
Enzyme-linked immunosorbent assay (ELISA) can detect more specific targets and is mainly used for MPO-ANCA and PR3-ANCA. (5 Likewise, in some studies, testing positive for ANCA by ELISA (MPO-ANCA and PR3-ANCA) has been associated with the worst prognosis in patients with SLE and lupus nephritis (LN). (6
Given these techniques and the incomplete or conflicting information linking ANCA detection and patterns with clini-cal outcomes, we sought herein to determine the frequency and clinical associations of ANCAs in patients with SLE from a cohort of Colombian SLE patients.
Materials and methods
We conducted a cross-sectional study of the serum of 74 patients with SLE evaluated between January 2018 and June 2019 in the Rheumatology service of the "Fundación Valle del Lili," a tertiary care hospital in Colombia. All patients signed an informed consent authorizing their participation in the study. Patient charts and immunological profiles were reviewed from clinical records. All patients met the SLE classification criteria of the Systemic Lupus International Collaborating Clinics (SLICC) 2012 and new SLE classification criteria of the American College of Rheumatology 2019. Renal involvement was defined as the presence of hematuria, proteinuria, or active urinary sediment. On the other hand, confirmed LN was grouped into proliferative and non-proliferative upon renal biopsy reports. Combinations such as III + IV, III + V, IV + V, and IV + VI were classified as proliferative.
ANCAs were measured by IIF with the NOVA Lite® ANCA kit (Inova Diagnostics, San Diego, CA, USA) using ethanol-fixed slides. A 1:10 dilution of each sample was used for screening, and those showing fluorescence were classified as positive, and were also classified by intensity, ranging from 1+ to 4+, according to manufacturer's specifications. The c-ANCA, p-ANCA, or atypical patterns were recorded for each case. Antibody specificities for myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA were determined using ELISA kits with an Alegria® automatic ELISA reader (Orgentec Diag-nostika, Mainz, Germany) with a cutoff of >5 U/mL. The assays were performed according to the manufacturer's instructions.
Statistical analysis
IIF and ELISA results were compared with the clinical char-acteristics, immunological profiles, and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) assigned according to IIF results. Normality distribution data were evaluated using the Kolmogorov-Smirnov test. Continuous variables are shown as medians (interquartile range, IQR) or means (± standard deviation). Categorical variables are shown as frequencies and percentages and were compared by chi-squared test or Fisher's test as appropriate. The Mann-Whitney test was used for continuous variables. All p values were two-tailed, and p values<0.05 were considered statistically significant.
Results
ANCA by IIF
Seventy-four patients with SLE (94% women) were included, with a median age of 30 years (IQR 21-40).
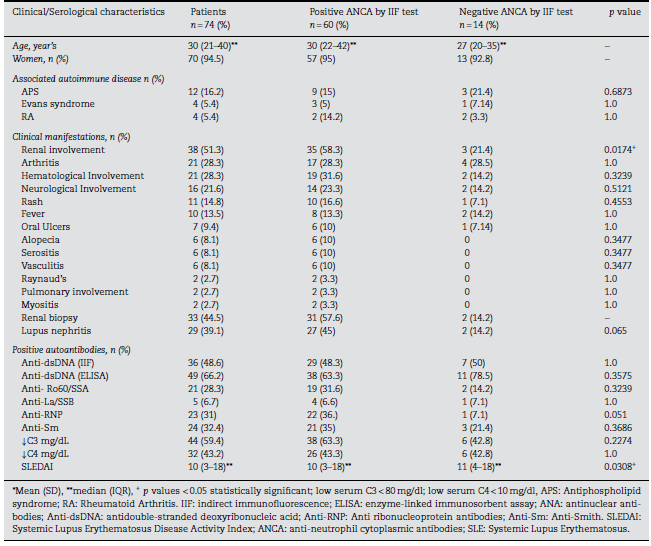
Sixty (81.1%) patients showed ANCA positivity by IIF of ethanol-fixed slides. The majority of patients (n=31) pre-sented a fluorescence intensity of 4+, followed by fifteen with 3+, six with 2+, and 8 with 1+. Fifty-eight (96.6%) of these 60 patients presented the p-ANCA pattern, while only two (3.33%) displayed the c-ANCA pattern. None of the patients exhibited an atypical pattern. The association between clinical and sero-logical characteristics of patients with SLE according to their ANCA positivity is shown in Table 1. Renal involvement was found in 35 cases (58.3%) at inclusion. The median score of SLEDAI was 10 (IQR: 3-18). ANCA positivity by IIF was associated with renal involvement (p = 0.0174) and lower values of SLEDAI (p = 0.0308), compared to a median score of 11 in those with negative ANCAs by IIF; although there is a minimum dif-ference between both scores, the statistical significance might be attributable to the number of patients compared (60 vs. 14)(Table 1).
Table 1 ANCA test results by IIF and presence of clinical characteristics and other autoantibodies in SLE patient
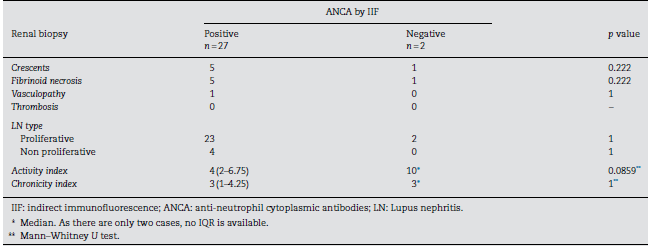
In terms of LN, twenty-nine patients (39.2%) had the diagnosis confirmed by renal biopsy. Of those, 27 presented positive IIF test results. We then evaluated if there was any relation between ANCAs positivity and crescents, fibri-noid necrosis, vasculopathy, thrombosis; LN type; activity, or chronicity index, but we did not find any significant result (Table 2).
Moreover, twenty-five patients with ANCAs by IIF of 3+ or 4+ did not have LN at the time of the blood sample. Of them, 17 had subsequent medical records of control appointments with a rheumatologist, of which three patients did develop LN; two of them had the confirmatory renal biopsy one month and 21 months after the blood sample, respectively. On the other hand, 14 did not develop LN by the last follow-up avail-able, which were 21 (IQR 4.5-28.5) months apart from the blood sample.
Discussion
We carried out a cross-sectional study in 74 patients with SLE to evaluate the presence of ANCAs by IIF and ELISA. Because only one patient was positive by ELISA, we compared clinical and serological features of those with ANCA-positive IIF results. The high discrepancy between IIF and ELISA tests may result from different factors.
For IIF, ethanol is the most frequently used fixative agent. This technique causes solubilization of the granule membranes, allowing mobilization of their content. Cell changes can lead to the recognition of different antigens of several granulocyte components other than MPO or PR3, including lactoferrin, cathepsin G, elastase, lysozyme, bacterial permeability-increasing protein, catalase, α-enolase, lamin B1, among others. (4,5,7 By contrast, the use of the cross-linking fixative formaldehyde prevents this redistribution6 The use of ethanol or formalin as fixative agents allows the distinction of true p-ANCA or c-ANCA patterns from those directed against other neutrophil targets. Looking for other antigen specificities by ELISA for routine purposes is not recommended. (8
In the same way, ANAs positivity can interfere with ANCAs, yielding a p-ANCA pattern on the ethanol-fixed neutrophil substrate. The use of formalin-fixed neutrophils overcomes this problem, helping to distinguish similar fluoroscopic staining that occurs due to the presence of ANAs.9,10 Herein, however, we used only ethanol as a fixative which could have caused the high proportion of positive ANCAs in our sample. The search for other antigen specificities or different fixation techniques may be pursued in other studies.
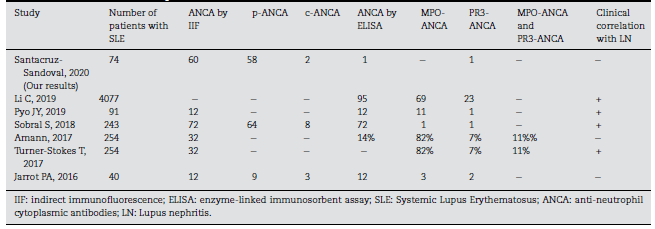
ANCAs detection may play a role in patients with SLE and LN. (11 Currently, different studies show an association with worse renal outcomes and poor prognosis in patients with LN and ANCAs positivity (Table 3). (12 The presence of ANCAs may, therefore, correlate with more severe clinical outcomes, his-tological necrosis, and crescentic glomerulonephritis. Indeed, ANCAs are directly implicated in the pathogenesis of this form of glomerular injury and are thought to activate cytokine-primed neutrophils and monocytes expressing ANCA-PR3 and MPO on their surfaces. (13
Moreover, some studies found an association between ANCAs and LN's classification of the International Society of Nephrology (ISN)/Renal Pathology Society. (14 These findings show that positive ANCAs serology in LN was associated with LN's class IV-S histological and clinical phenotype. (15,16 In other studies, MPO-ANCA mainly contributed to the chronicity index of LN17 and was positively correlated with class IV LN. (18) Nevertheless, Olson et al. found that elevated dsDNA antibody was not associated with elevated MPO-ANCA levels. (19 Inter-estingly, our results did not show any of the statements found by the mentioned authors; instead, no relation was found between histological findings frequently seen in the presence of ANCA, nor associations with any LN classification, neither with activity or chronicity indexes. Pan et al. and Wang et al. had similar results regarding LN classification, indexes, presence of crescents and necrosis; as no difference was found between ANCA-positive vs. ANCA-negative patients in their cohorts. (20,21
Some authors maintain that no significant clinical or pathological differences in SLE patients are associated with the presence or absence of ANCAs, and no significant correlation is present between clinical features and the status of either p-ANCA or c-ANCA. (22 In one study, the presence of ANCAs in patients with SLE rarely affected the clinical manifestations of SLE, (23 and in another, there was no association of ANCA with renal involvement. (24 In addition, Pan et al. studied SLE 120 patients, of which 45 had positivity in p-ANCA by IIF, 75 were negative for p-ANCA and none of them was positive in ELISA techniques; in this case, they did find more occurrence of LN and more Raynoud syndrome in patients with positive p-ANCA vs those that were negative. (20
Despite this, it is essential to note that ANCAs in LN are associated with a higher SLE disease activity index score. (25 Li et al. found that ANCA-positive LN patients had higher SLEDAI scores, which might be associated with higher hematuria because of aggravated kidney injuries caused by ANCAs. (26) Herein, we obtained a significant correlation between SLEDAI index and renal outcomes (hematuria, proteinuria, and the presence of urinary sediment).
Other studies have shown that ANCAs can also be detected in healthy subjects and in several inflammatory or autoimmune diseases where its pathophysiological significance remains debatable. (27 Nevertheless, ANCAs is an independent risk factor for poor renal outcomes in LN patients. (28
Conclusion
In our study, we observed that most patients with SLE tested positive for ANCAs by IIF, but this was combined with PR3 specificity in only one case. This finding may be due to the presence of other target antigens. We found a statistically significant correlation between SLEDAI index and renal outcomes in patients with SLE who are positive for ANCAs, but not with LN classes or indexes. Further studies are needed to determine the specificity of ANCAs detected by IIF in our patients and others with SLE.